Explain the fact that in alkyl aryl ethers, alkoxy group :
(i) activates the benzene ring towards electrons subsitution.
(ii) directs the incoming substitudents word ortho and para positions in the ring.
(i) The alkoxy group (R⋅⋅O⋅⋅−) with lone electron pains on the oxygen atom activates the ortho and para positions in the ring by +M(or+R) effect as shown below:
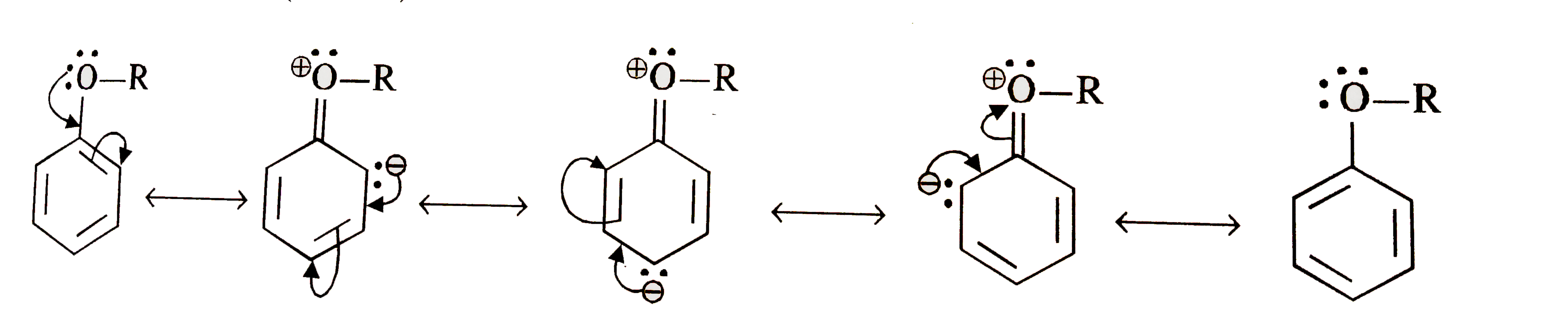
As the ortho group directs the incoming in the ring become paints of high electron density, the electrophiles prefer to attack these positions.
(ii) The alkoxy group directs the incoming group which is an electrophile towards the ortho and para positions in the ring. As a result , a mixture of osomeric products is formed. For details. consult section 24
Similar Questions
Explain the fact that in aryl alkyl ethers (i) the alkoxy group activates the benzene ring towards electrophilic substitution and (ii) it directs the incoming substituents to ortho and para position in benzene ring.
View SolutionExplain the fact that in aryl ethers, (i) the alkoxy group activates the benzene ring towards electrophilic substitution and (ii) it directs the incoming substituents to ortho and para positions in benzene ring.
View Solution
Knowledge Check
A group which deactivates the benzene ring towards electrophilic substitution but directs the incoming group towards o- and p- position is
A−NH2B−ClC−NO2D−C2H5A group which deactivates the benzene ring towards electrophilic substitution but which directs the incoming group principally to the o- and p-positions is
A−NH2B−ClC−NO2D−C2H5Which of the following group activates the benzene ring most towards electrophilic substitution
A−CHOB−NR2C−NHCOCH3D−NO2
Explain the fact that in ary alkyl ethers (i) the alkoxy group activates the benzene ring towards electrophilic substitution and (ii) it direct the incoming substituents to ortho - and para - positions in benzene ring.
View SolutionAlkoxy group attached to benzene ring is ortho and para directing. Justify.
View SolutionExplain how an OH group attached to a carbon in the benzene ring activates benzene towards electrophilic substitution.
View SolutionWhich of the following group(s) activate the benzene ring towards electrophilic substitution reaction.
View SolutionIf aromatic ring is substituted by more than one group then electrophilic aromatic substitution reaction take place according to more activating group. The group which donates electrons to aromatic ring knwn as activating group and which withdraw electrons from the ring is called electron withdrawing group. generally all lectron releasing groups activates benzene ring towards electrophilic substitution and electron withdrawing groups deactivates ring towards electrophilic substitutions.
Q. Find out major product of following reaction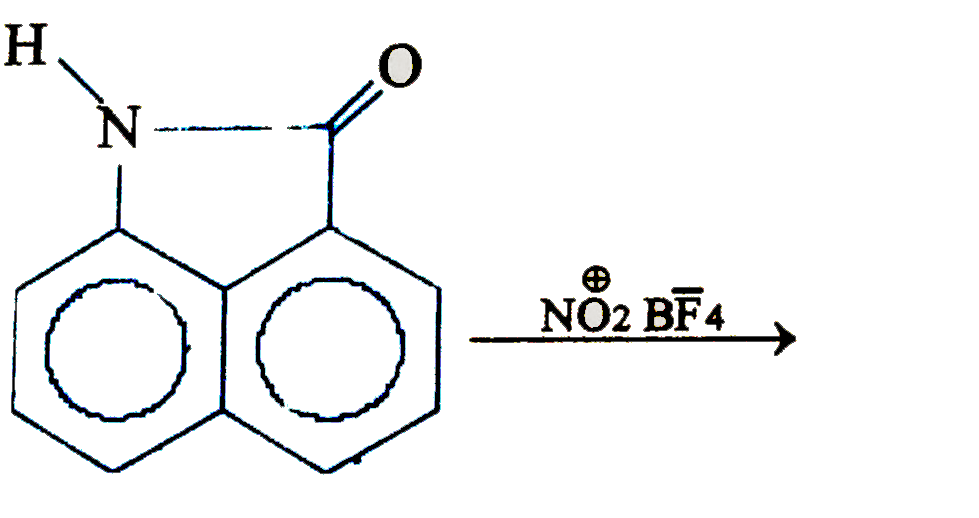
View Solution

