Assertion : Oxidation of toluene as well as ethyl benzene with KMnO4 gives benzoic acid.
Reason : Both toluene and ethyl benzene are more reactive than benzene towards electrophilic substitution reactions.
If both assertion and reason are correct and reason is correct explanation for assertion.
If both assertion and reason are correct but reason is not correct explanation for assertion.
If assertion is correct but reason is incorrect.
If assertion and reason are both incorrect.
The correct Answer is:C
(c) Correct explanation. Irrespective of the length of the alkyl side chain, oxidation occurs at more reactive benzylic car bon atom leading to the formation of benzoic acid.
Similar Questions
Knowledge Check
Assertion : Oxidation of toluene as well as ethyl benzene with KMnO4 gives benzoic acid.
Reason : Both toluene and ethyl benzene are more reactive than benzene towards electrophilic substitution reactions.AIf both assertion and reason are true, and reason is the true explanation of the assertion.BIf both assertion and reason are true, but reason is not the true explanation of the assertion.CIf assertion is true, but reason is false.DIf both assertion and reason are falseWhich of following species is less reactive than benzene in electrophilic substitution reaction
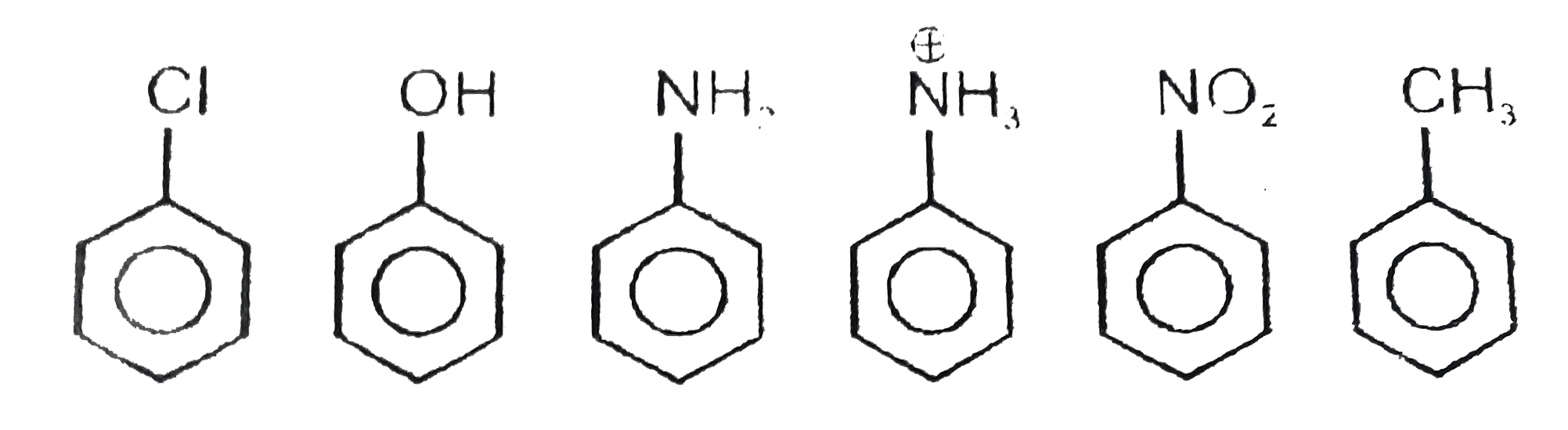
AanilineBbromobenzeneCnitrobenzeneDphenolWhich of the following group(s) activate the benzene ring towards electrophilic substitution reaction.
A−ClB C−O−O||C−CH3D−C≡..N
C−O−O||C−CH3D−C≡..N
Name the compound formed by the oxidation of ethyl benzene by KMnO4.
View SolutionDirective Influence Towards EAS(Electrophilic Substitution Reaction) ||Nitration OF Benzene
View SolutionAll the common m-directig group make the benzene ring towards electrophilic substitution reactions
View SolutionWhich of the following species is less reactive than benzene towards ring substitution reactions ?
View SolutionAssertion : Chlorobenzene is more reactive than benzene towards the electrophilic substitution reaction.
Reason : Resonance destabilizes the carbocation.
View Solution