Assertion : Phenol is more reactive than benzene towards electrophilic substitution reaction
Reason : In the case of phenol, the intermediate arenium ion is more stabilized by reasonance.
if both assertion and reason are true and reason is the correctexplanationof assertion.
if both assertion and reason are true but reason is not the correct explanationof asserton.
assertion is true but reason is false
both assertion and reason are false.
The correct Answer is:A
if both assertion and reason are true and reason is the correctexplanationof assertion.
Topper's Solved these Questions
- HYDROXY COMPOUNDS AND ETHERSBOOK - FULL MARKSCHAPTER - HYDROXY COMPOUNDS AND ETHERSEXERCISE - TEXTBOOK EVALUATION (ANSWER THE FOLLOWING QUESTIONS)
25 Videos
- HYDROXY COMPOUNDS AND ETHERSBOOK - FULL MARKSCHAPTER - HYDROXY COMPOUNDS AND ETHERSEXERCISE - Evaluate Yourself
14 Videos
- ELECTRO CHEMISTRYBOOK - FULL MARKSCHAPTER - ELECTRO CHEMISTRYEXERCISE - ADDITIONAL QUESTIONS -5- MARK QUESTIONS
15 Videos
- IONIC EQUILIBRIUMBOOK - FULL MARKSCHAPTER - IONIC EQUILIBRIUMEXERCISE - ADDITIONAL QUESTIONS (5 MARK QUESTION)
15 Videos
Similar Questions
Identify the product (s) is/ are formed when 1-methoxy proane is heated with excess HI. Name the mechanism involved in the reaction
View SolutionClassify the following alcohols as 1∘,2∘,and3∘ and give their IUPAC names.
(a) CH3−CH2−CH(OH)CH2−Br∣C(CH3)2 (b) (C2H5)3COH
(C ) CH2=C(CI)−CH(OH)CH3 (d)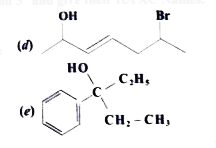
View SolutionHow would you measure the conductivity of ionic solutions?
View SolutionDifferentiate Lewis acids and Lewis bases.
View SolutionPhenol is more reactive than benzene towards electrophilic substitution reaction.
In case of Phenol, the intermediate carbocation is more resonance stabilised.
View SolutionAssertion: Phenol is more reactive than benzene towards electrophilic reactions.
Reason : The +R effect of OH group increases the electron density on benzene nucleus.
View SolutionAssertion : Phenol is more reactive than benzene towards electrophilic substitution.
Reason : OH group in phenol is ortho and paradirecting in nature.
View SolutionStatement-I: Phenol is more reactive than benzene towards electrophilic substitution reaction. Because Statement-II: In the case of phenol, the intermediate carbocation is more reasonance stablized.
View SolutionStatement-I: Phenol is more reactive than benzene towards electrophilic substitution reaction.
Because
Statement-II: In the case of phenol, the intermediate carbocation is more reasonance stablized.
View SolutionStatement 1: Phenol is more reactive than benzene towards electrophilic substitution reaction. Statement 2: In the case of phenol, the intermediate carbocation is more resonance stabilized.
View SolutionAssertion : Chlorobenzene is more reactive than benzene towards the electrophilic substitution reaction. Reason : Resonance destabilizes the carbocation.
View SolutionAssertion: Phenol is more reactive than benzene towards electrophilic substitution reaction.
Reason: In the case of phenol, the intermediate arenium ion is more stabilized by resonance.
View SolutionGive reason for the following: (a) p-nitrophenol is more acidic than o-nitrophenol. (b) Phenol is more reactive towards electrophilic substitution reaction than benzene.
View SolutionAssertion: Phenol is more reactive than benzene towards electrophilic substitution reaction.
Reason: In the case of phenol, the intermediate arenium ion is more stabilized by resonance.
View SolutionAssertion(A): Phenol is more reactive towards electrophilic substitution reaction.
Reason (R): In the case of phenol. the intermediate carbo cations is more resonance stabilized.
View Solution

