Arrange in order of increasing I.P.
A
Mg<Al<Si<P
B
Al<Si<P<Mg
C
Si<P<Mg<Al
D
Al<Mg<Si<P
Video Solution
Text Solution
The correct Answer is:D
All of these elements belong to 3rd period. Mg(Z=12) , Al(Z=13), Si(Z=14),P(Z=15), Mg(1s22s22p63s2) and P(1s22s22p6)3s23p3) due to fully fille dand haf fille subshells have high I.E1.I.E1 in general increases in a period from left to right. thus, correct ordre of I.E1 is
Al(I.E1=577)<Mg(I.E1=738)<si(I.E1 values are in kJ mol−1)
|
Similar Questions
Knowledge Check
Arrange in the order of increasing acidic strength :
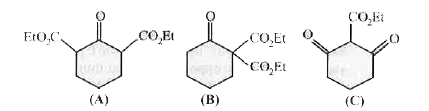 AA<B<CBA<C<BCB<A<CDB<C<A
AA<B<CBA<C<BCB<A<CDB<C<AArrange in the order of increasing acidic strengths.
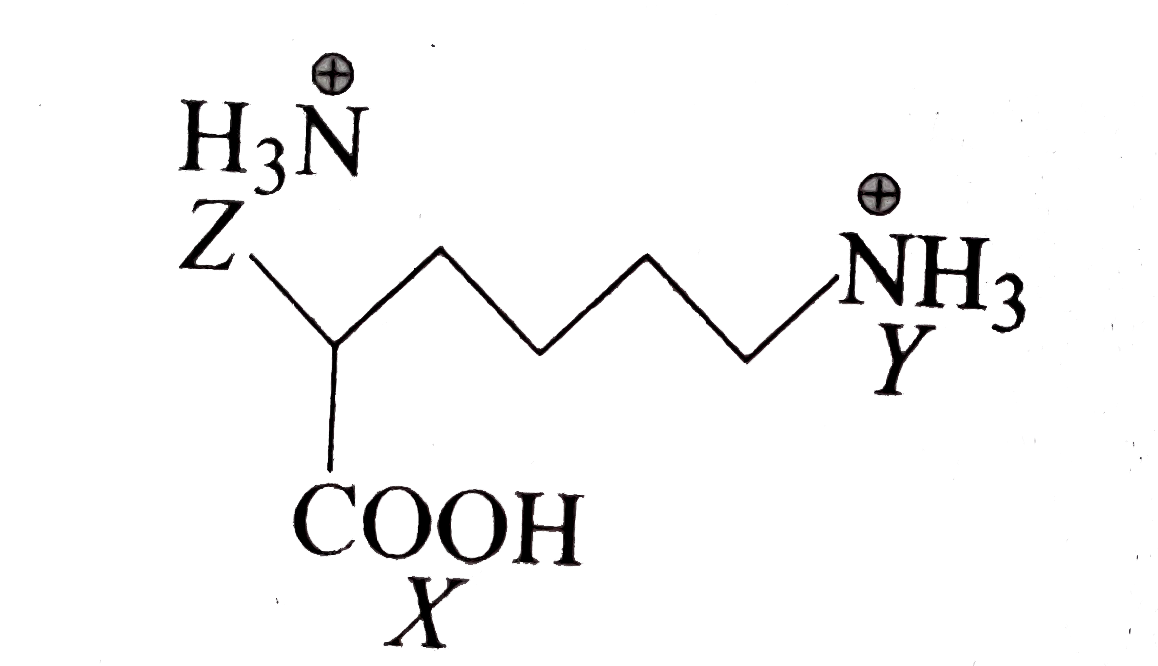 AX>Z>YBZ<X>YCX>Y>ZDZ>X>Y
AX>Z>YBZ<X>YCX>Y>ZDZ>X>Y
Arrange in order of increasing acid strengthAX>Z>YBZ<X>YCX>Y>ZDZ>X>Y
The chemical elements are arranged in the order of increasing electronegativities in the sequence:
View Solution
Arrange in order of increasing acid strength
View SolutionFour compounds, Toluene (I), o-dichlorobenzene (II), m-dichlorobenzene (III) and p-dichlorobenzene (IV) are arranged in the order of increasing dipole moment. The correct order is
View SolutionSoil particle arranged in order of increasing size are:
View SolutionIn which list are the ions arranged in order of increasing size?
View Solution

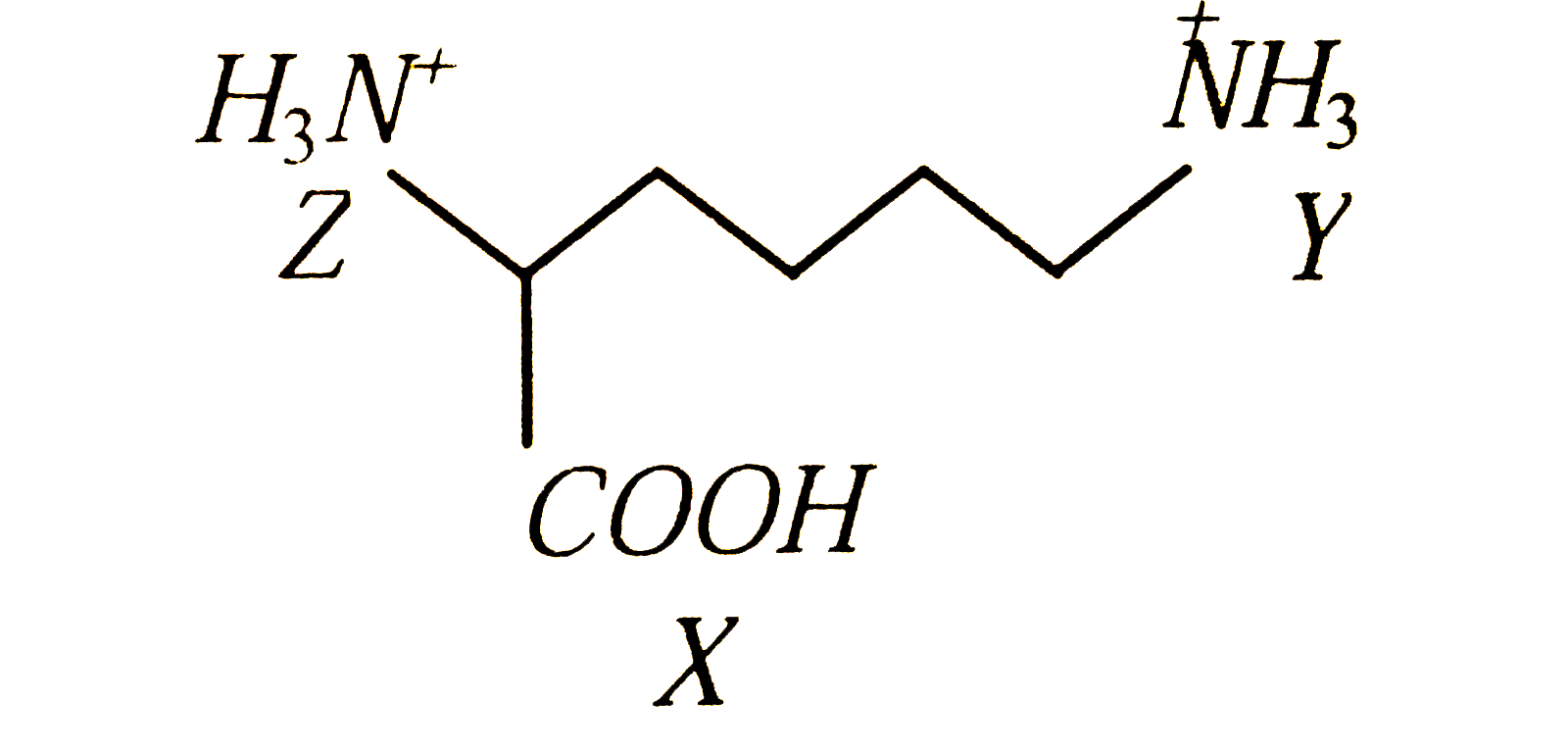
_CHE_E06_190_Q01.png)