Homogeneous mixing and compressibiltiy both result from the fact that the molecules are far apart in gases. Mixting occurs because indicidual gaseous molecules have little interaction with their neighbours, assuming that no reaction taken place, the chemical identites is possible in gases because only about 0.1% of the volume of a typical gas is taken up by the molecules themselves under normal circumstances, the remaining 99.9% is empty space
Compressibility factor of a gas is given by
1PVR
PVR2
RPV
PVnRT
The correct Answer is:D
Topper's Solved these Questions
- STATE OF MATTER (GASEOUS AND LIQUID STATE)Book:DINESH PUBLICATIONChapter:STATE OF MATTER (GASEOUS AND LIQUID STATE)Exercise:Matrix Match type MCQs
Explore 2 Videos
- STATE OF MATTER (GASEOUS AND LIQUID STATE)Book:DINESH PUBLICATIONChapter:STATE OF MATTER (GASEOUS AND LIQUID STATE)Exercise:Integer type question
Explore 4 Videos
- STATE OF MATTER (GASEOUS AND LIQUID STATE)Book:DINESH PUBLICATIONChapter:STATE OF MATTER (GASEOUS AND LIQUID STATE)Exercise:Selected straight objective types MCQs
Explore 58 Videos
- S-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS )Book:DINESH PUBLICATIONChapter:S-BLOCK ELEMENTS (ALKALI AND ALKALINE EARTH METALS )Exercise:Assertion reason
Explore 12 Videos
- STATES OF MATTER (SOLID STATE CHEMISTRY)Book:DINESH PUBLICATIONChapter:STATES OF MATTER (SOLID STATE CHEMISTRY)Exercise:ULTIMATE PREPARATORY PACKAGE
Explore 21 Videos
Similar Questions
Here each question contains statements given in two colums which have to be matched.
Statements in column I are labelled as A, B, C and D whereas the statement is column II are labelled as p, q, r and r. The answers to these questions are to be appropriately bubbled as illustrated in the following example
If the correct matches are A−p,A−s,B−q,B−r,C−p,C−q, and D−p then correctly labelled 4×4 matrix should look like the following
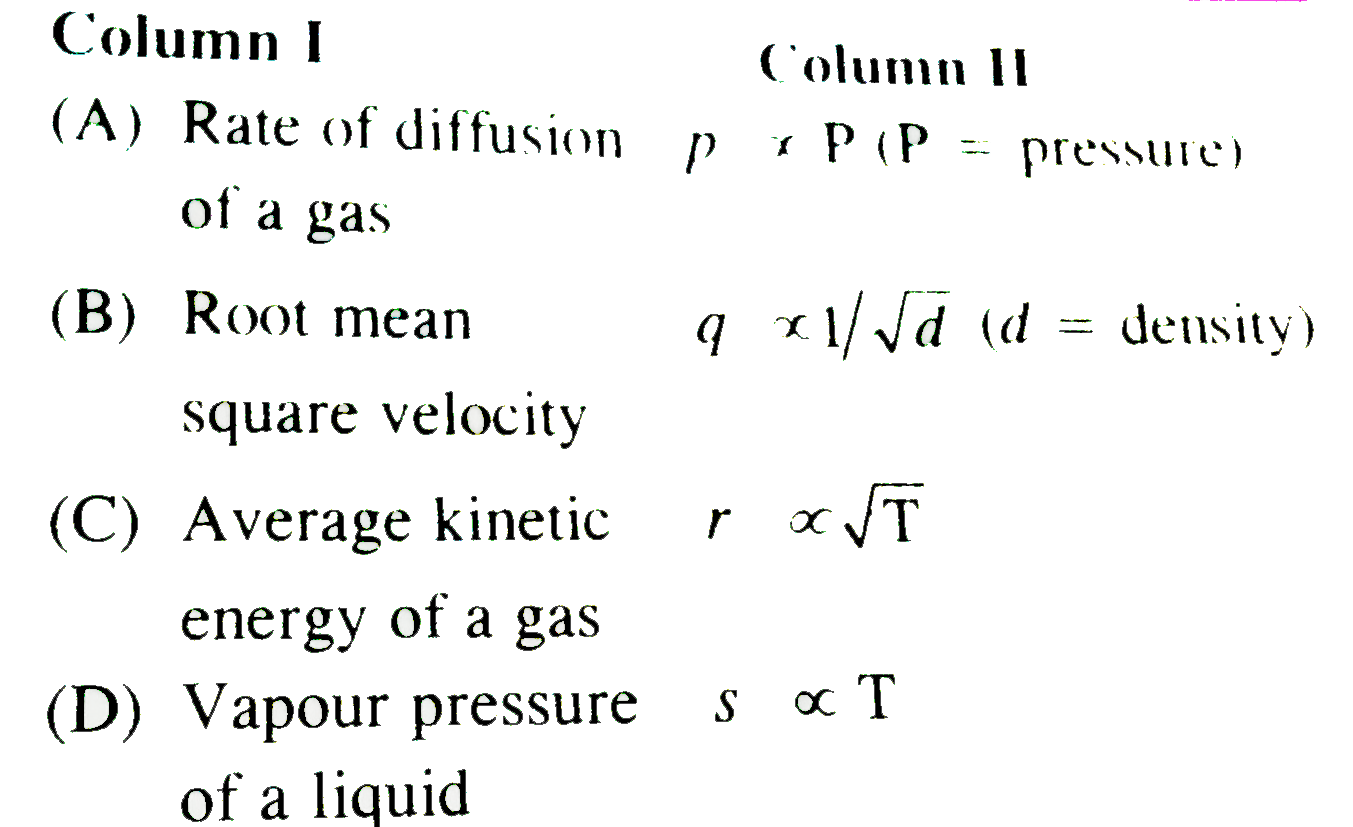
View SolutionAt 400K, the root mean square (rms) speed of a gas X (molecular weight = 40) is equal to the most probable speed of gas Y at 60 K. The molecular weight of the gas Y is.
View SolutionAccording to Boyle's law
View SolutionAssertion (A): Lithium chloride is predominantly covalent compound.
Reason (R ): electronegativity difference between Li and CI is small.
View SolutionWhich of the following is not a Bravais lattice ?
View SolutionIn kinetic theory of gases, only translational motion of molecules is considered because:
View SolutionHomogeneous mixing and compressibiltiy both result from the fact that the molecules are far apart in gases. Mixting occurs because indicidual gaseous molecules have little interaction with their neighbours, assuming that no reaction taken place, the chemical identites is possible in gases because only about 0.1% of the volume of a typical gas is taken up by the molecules themselves under normal circumstances, the remaining 99.9% is empty space
Assume molecules are spherical of radius 1Å, volume occupied by molecules in one mole of a gas at NTP is
View SolutionHomogeneous mixing and compressibiltiy both result from the fact that the molecules are far apart in gases. Mixting occurs because indicidual gaseous molecules have little interaction with their neighbours, assuming that no reaction taken place, the chemical identites is possible in gases because only about 0.1% of the volume of a typical gas is taken up by the molecules themselves under normal circumstances, the remaining 99.9% is empty space
For H2 and He, force of attraction is negligible, hence, compressibility factor is
View SolutionGases excert pressure on the walls of the container , because the gas molecules
View SolutionGases deviate from the ideal gas behaviour because their molecules
View SolutionHomogeneous mixing and compressibility both result from the fact that the molecules are far apart in gases. Mixing occurs because individual gaseous molecules have little interaction with their neighbours and, assuming that no reaction takes place, the chemical identities of those neighbours are irrelevant. Compressibility is possible in gases because only about 0.1% of the volume of a typical gas is taken up by the molecules themselves under normal circumstances, the remaining 99.9% is empty space.
Compressibility factor of a gas is given by :
View SolutionHomogeneous mixing and compressibility both result from the fact that the molecules are far apart in gases. Mixing occurs because individual gaseous molecules have little interaction with their neighbours and, assuming that no reaction takes place, the chemical identities of those neighbours are irrelevant. Compressibility is possible in gases because only about 0.1% of the volume of a typical gas is taken up by the molecules themselves under normal circumstances, the remaining 99.9% is empty space.
Assume molecules are spherical of radius 1A . Volume occupied by molecules in one mole of a gas at NTP is :
View SolutionHomogeneous mixing and compressibility both result from the fact that the molecules are far apart in gases. Mixing occurs because individual gaseous molecules have little interaction with their neighbours and, assuming that no reaction takes place, the chemical identities of those neighbours are irrelevant. Compressibility is possible in gases because only about 0.1% of the volume of a typical gas is taken up by the molecules themselves under normal circumstances, the remaining 99.9% is empty space. For H_(2) and He, force of attraction is negligible, hence compressibility factor is :
View SolutionThe kinetic theory of gases presumes the collisions between the molecules to be perfectly elastic because the collisions between molecules of a gas:
View SolutionGases excert pressure on the walls of the container , because the gas molecules
View Solution

