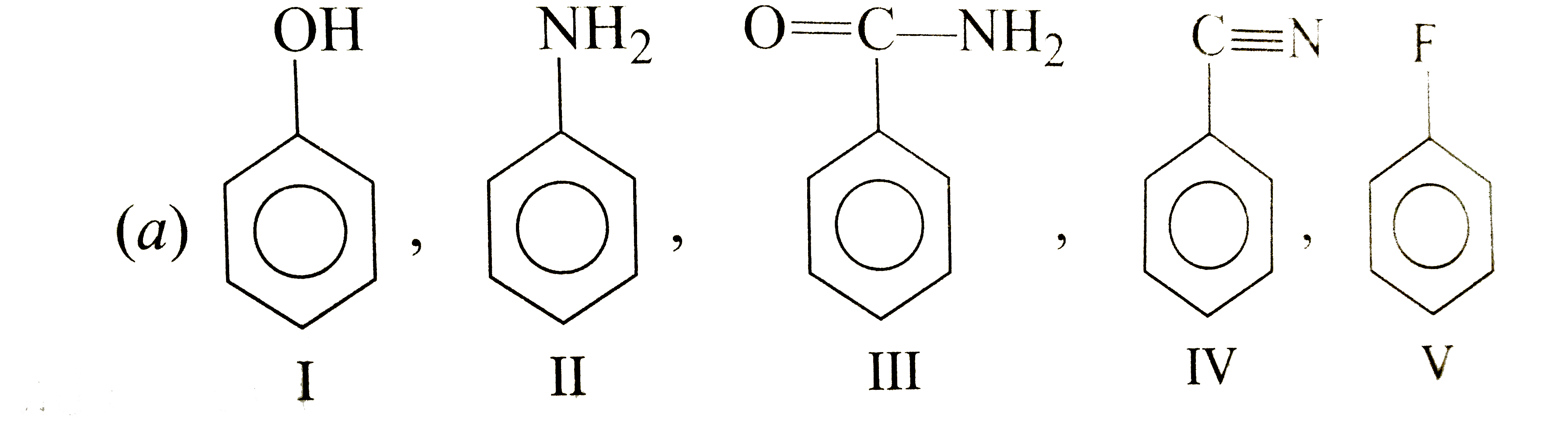
Arrange the following compounds in order of decreasing acidity:
A
III>I>II>IV
B
IV>III>I>II
C
II>IV>I>III
D
I>II>III>IV
Text Solution
The correct Answer is:A
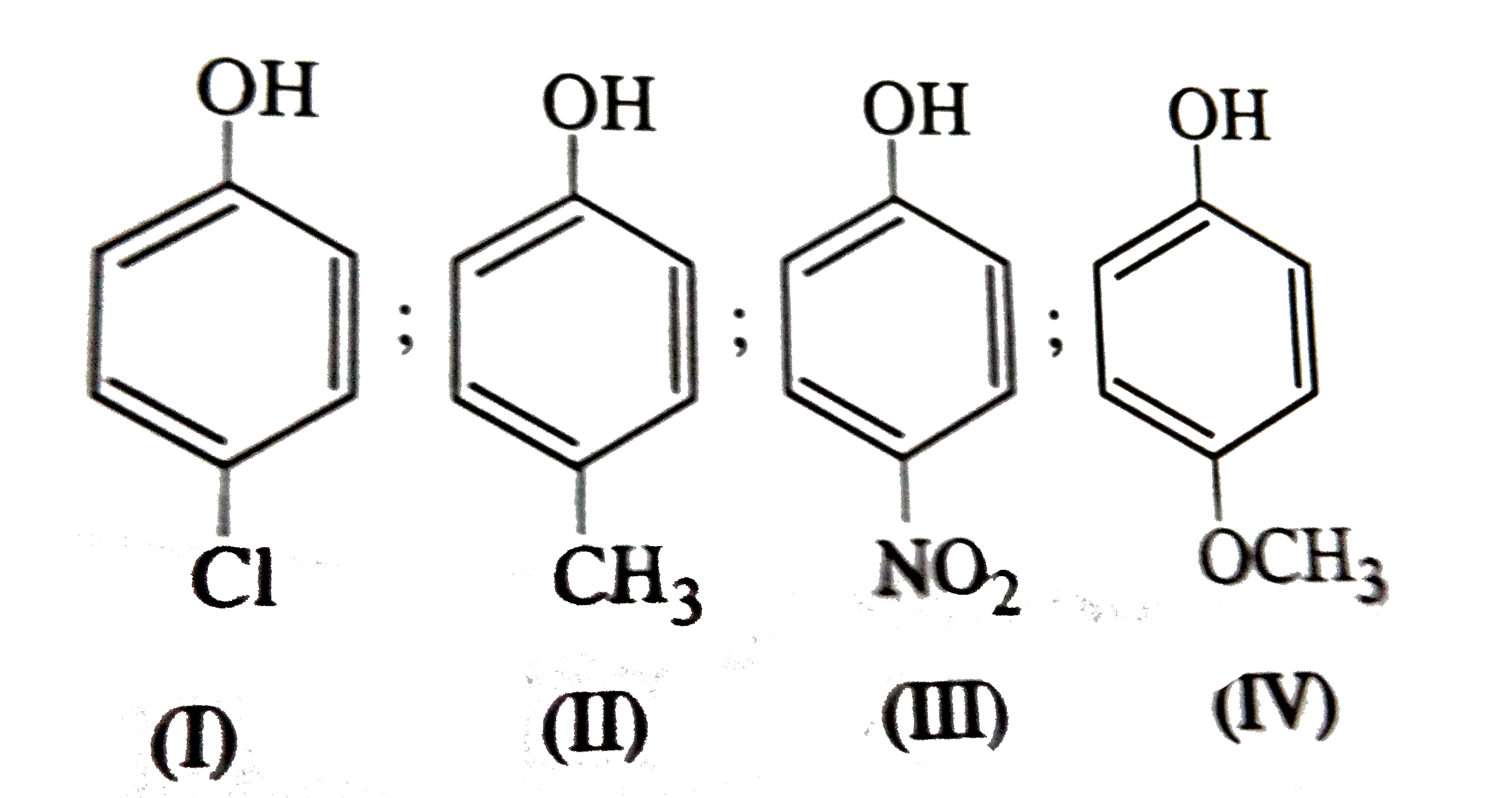
Electron withdrawing groups will increase the acidic character while electron releasing group will decrease the acidic character. Further R- effect is stronger than I-effect.
Here, −Cl has −I effect and +R effect,
−CH3 has weak +I effect,
−NO2 has −I and −R effect and
−OCH3 has −I and +R effect.
Thus correct order is :
III>I>I>IV.
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Arrange the following c ompounds in order of decreasing acidity:
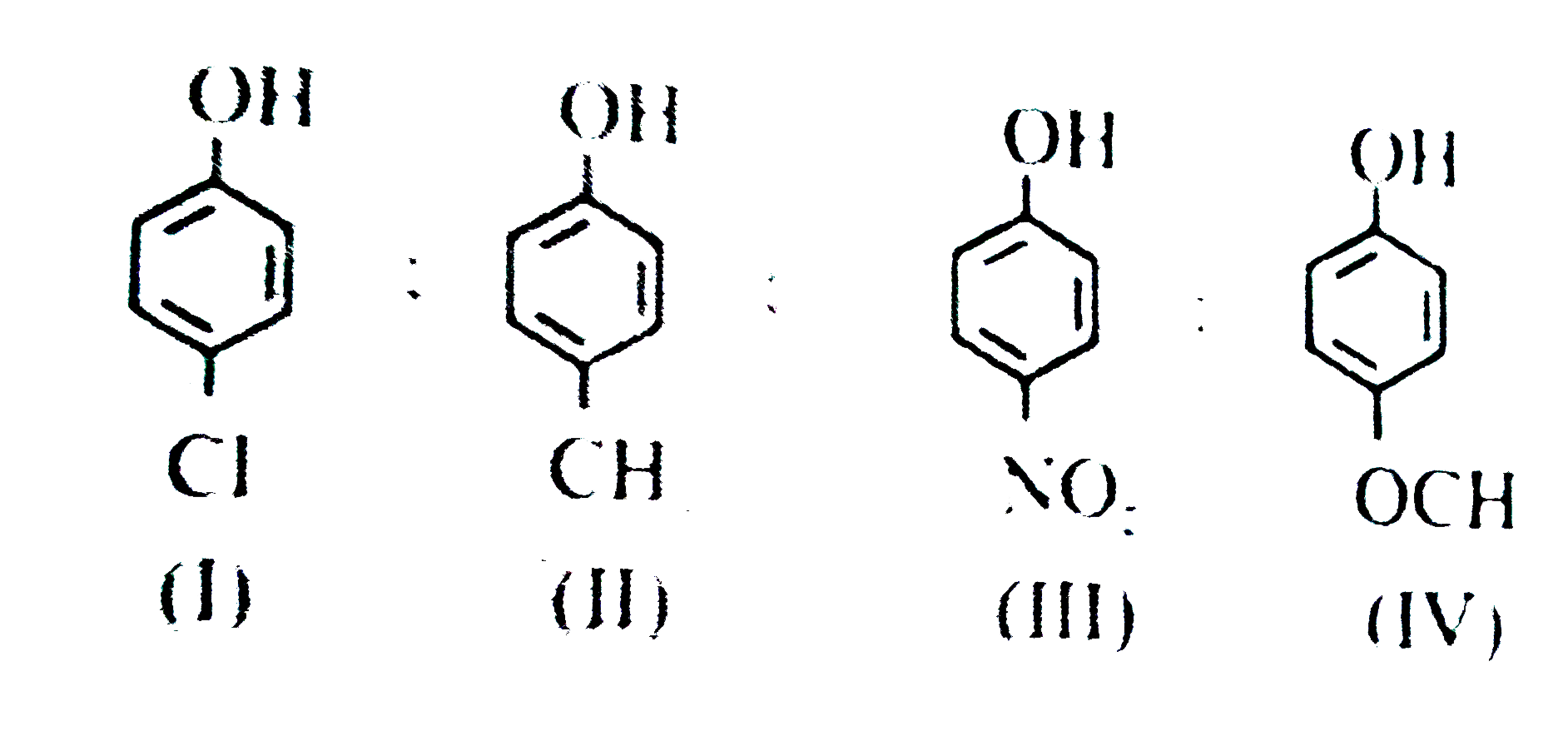 AIIgtIVgtIgtIIIBIgtIIgtIIIgtIVCIIIgtIgtIIgtIVDIVgtIIIgtIgtII
AIIgtIVgtIgtIIIBIgtIIgtIIIgtIVCIIIgtIgtIIgtIVDIVgtIIIgtIgtIIArrange the following compounds in order of decreasing acidic strength.
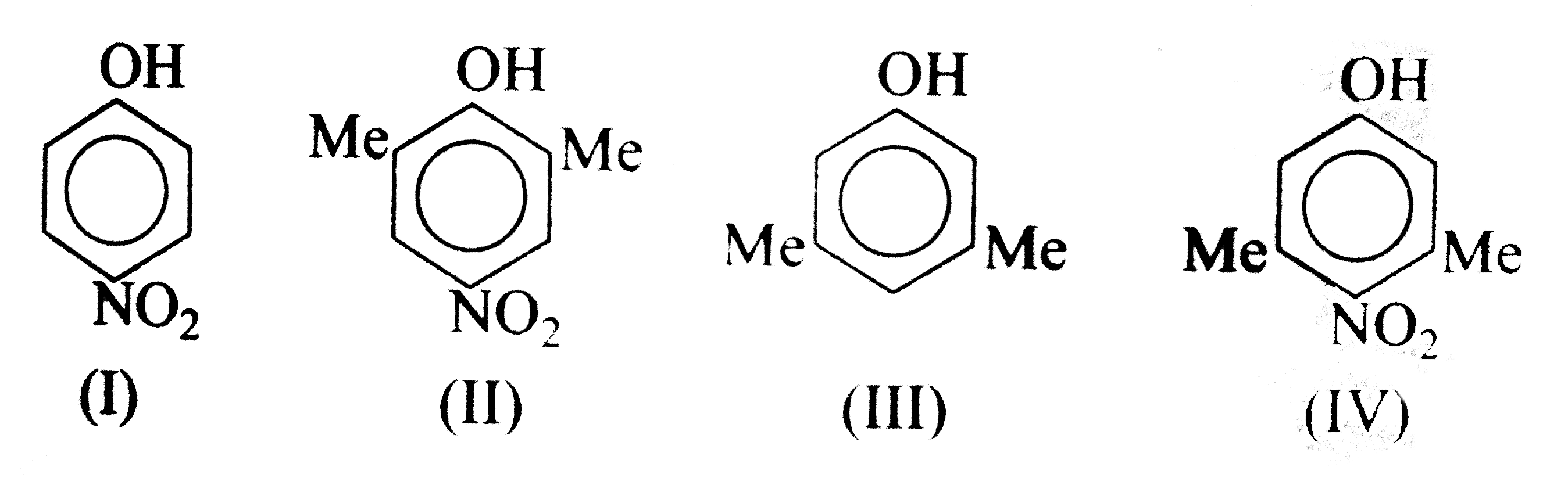 AI>II>III>IVBIII>I>II>IVCI>III>II>IVDII>IV>I>III
AI>II>III>IVBIII>I>II>IVCI>III>II>IVDII>IV>I>IIIArrange the following compounds in order of decreasing acidic strength.
 AI>II>III>IVBIII>I>II>IVCIV>III>I>IIDII>IV>I>III
AI>II>III>IVBIII>I>II>IVCIV>III>I>IIDII>IV>I>III
Arrange the following compounds in decreasing order of acidity :
H2O,ROH,HC≡CH
View SolutionArrange the following compounds in order of: Decreasing SN1 reaction rate:
View SolutionArrange the following compounds in the order of decreasing acidity.
I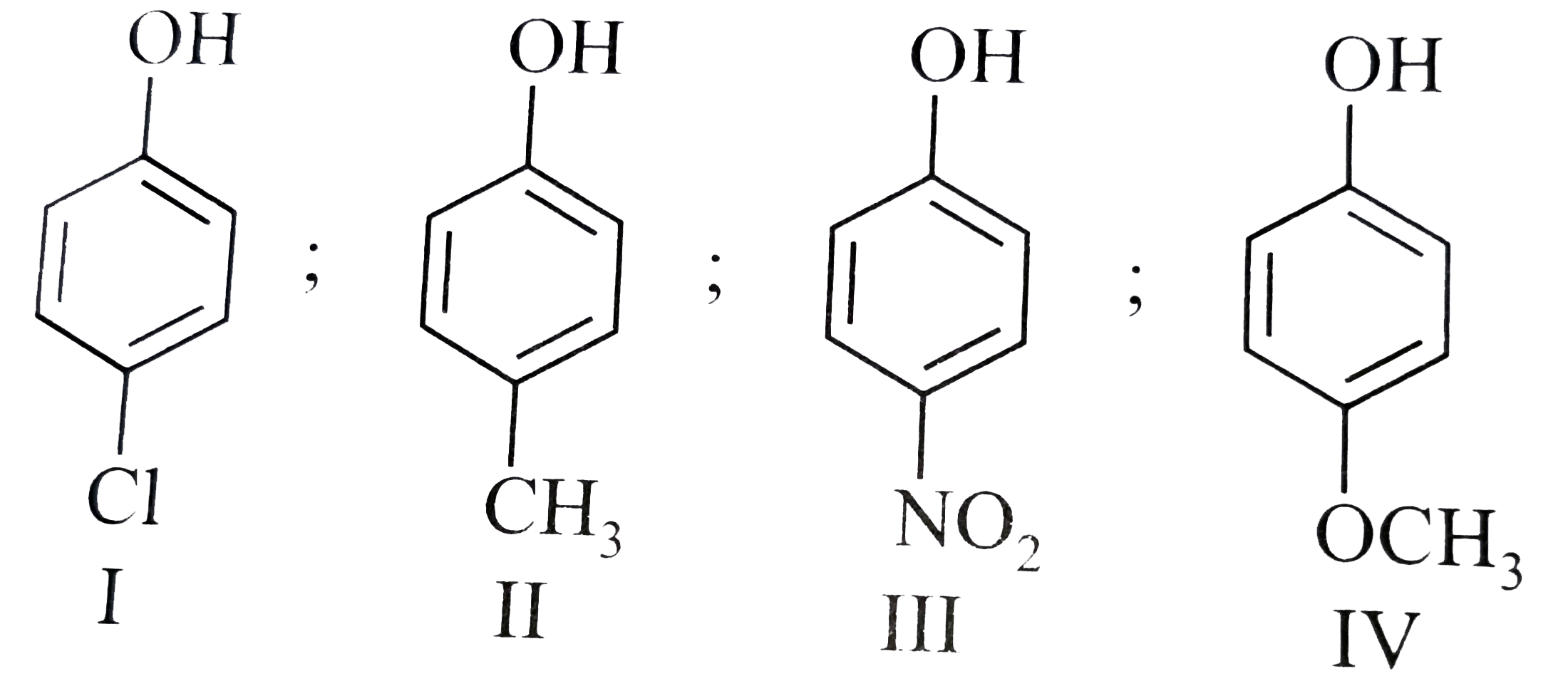 II
II  III
III  IV
IV 
View SolutionArrange the following compounds in decreasing order of acidity :
CH3CH2OH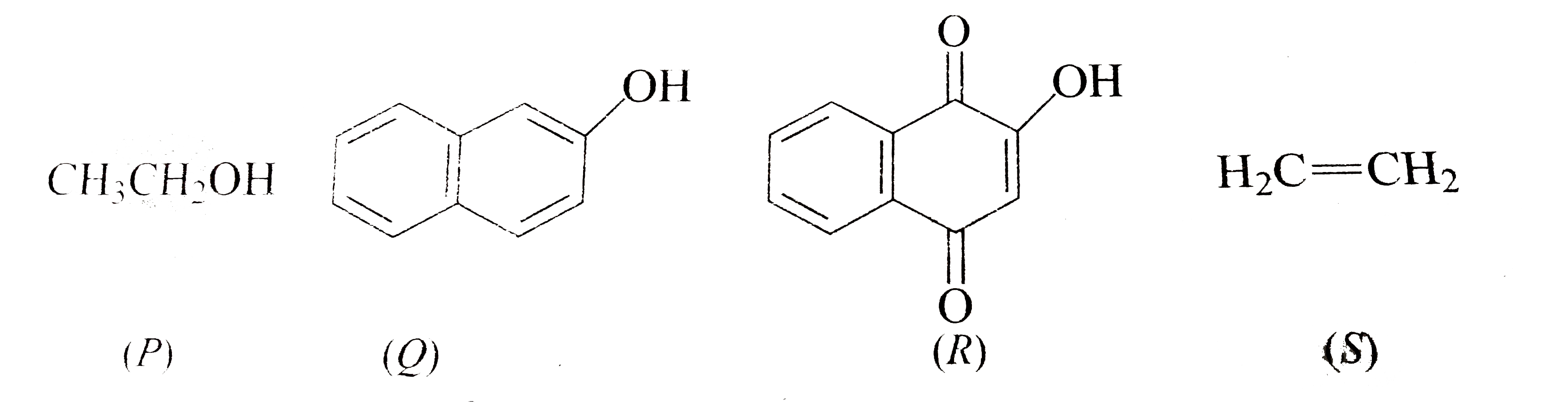
H2C=CH2
View SolutionThe correct sequence of arrangement of the following compounds in order of decreasing oxidation numbers of iodine is
View Solution