Which of the following is strongly acidic ?
A
Phenol
B
o-Cresol
C
p-Nitrophenol
D
p-Cresol
Text Solution
The correct Answer is:C
Electron withdrawing groups such as −NO2, group withdraws electrons, disperses the negative charge, stabilizes the phenoxide ion w.r.t. phenol and this increase the acidic strength.
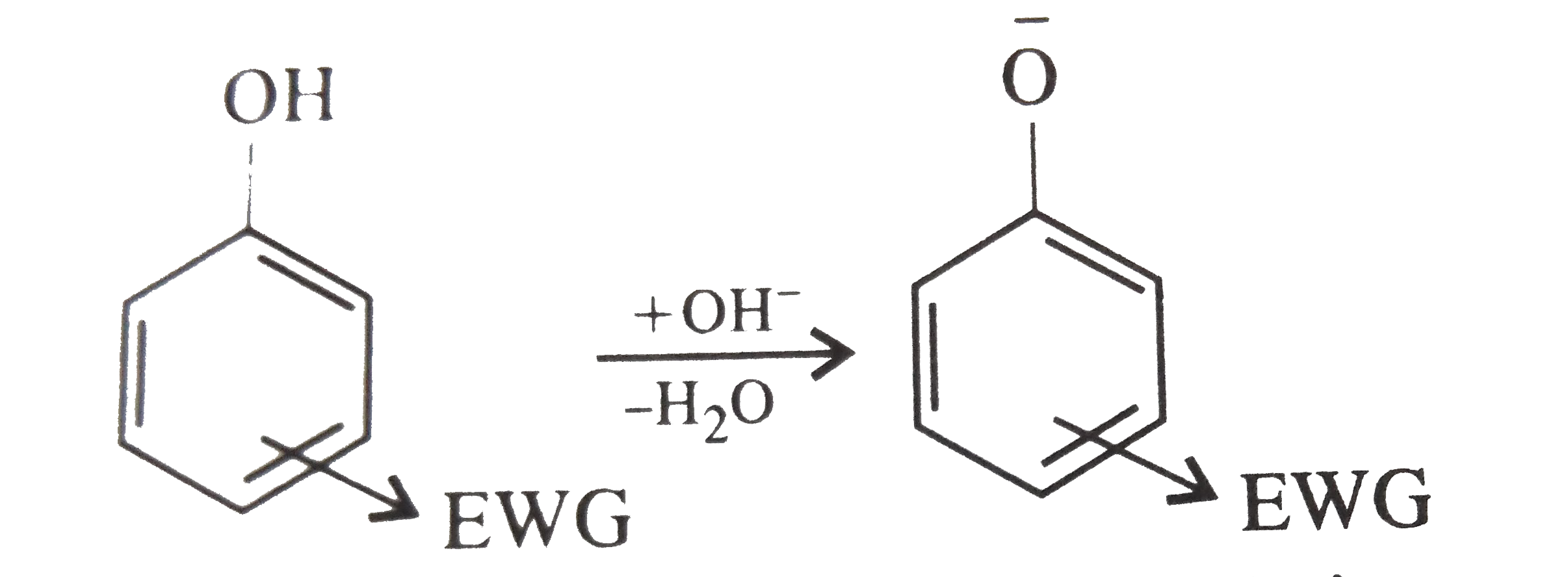
Electron-donating group such as −CH3 destabilizes the phenoxide ion by intensifying the negative charge relative to phenol. This tend to decrese the acidic strength of phenols
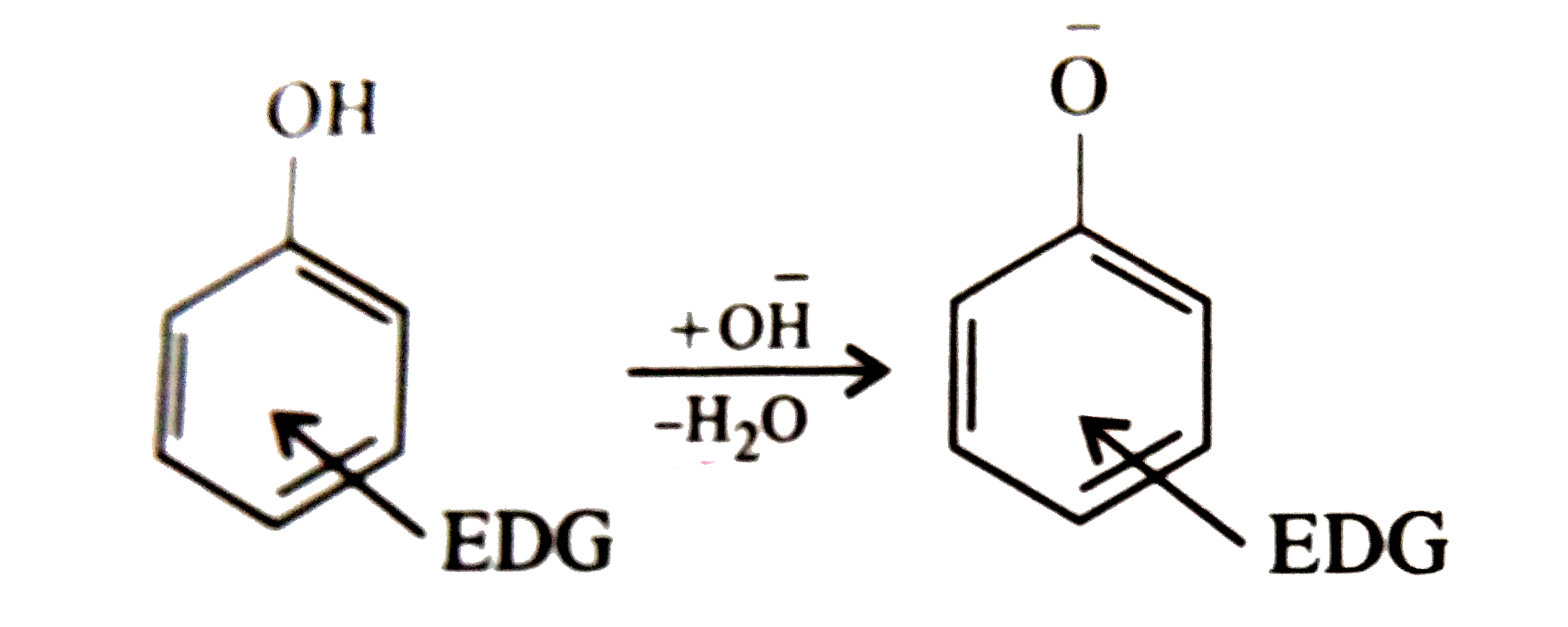
In cresols presence of −CH3 group decreases the acidic character while in nitrophenol presence of −NO2 increases the acidic character. Thus p-nitrophenol is most acidic.
|
Topper's Solved these Questions
- ALCOHOLS AND PHENOLSBook:DINESH PUBLICATIONChapter:ALCOHOLS AND PHENOLSExercise:Selected Straight Objective Type MCQs
Explore 55 Videos
- ALCOHOLS AND PHENOLSBook:DINESH PUBLICATIONChapter:ALCOHOLS AND PHENOLSExercise:Linked Comprehension Type MCQs
Explore 8 Videos
- ALCOHOLS AND PHENOLSBook:DINESH PUBLICATIONChapter:ALCOHOLS AND PHENOLSExercise:Ultimate Preparatory Package
Explore 20 Videos
- APPENDICESBook:DINESH PUBLICATIONChapter:APPENDICESExercise:Multiple choice
Explore 76 Videos
Similar Questions
Knowledge Check
Which of the following is strongly associated with cephalisation ?
AAsymmetryBRadial symmetryCBilateral symmetryDBiradial symmetryWhich of the following is strongly hydrated in aqueous solution ?
ALi⊕BNa⊕CK⊕DCs⊕Which of the following oxides is strongly basic?
AB2O3BAl2O3CGa2O3DTl2O3
Which one of the following strongly threatens biodiversity ?
View SolutionWhich one of the following strongly threatens biodiversity ?
View SolutionWhich of the following oxides is strongly basic?
View SolutionWhich of the following evidences strongly support the origin of amphibians from fishes?
View SolutionWhich of the following is water-soluble and strongly basic?
View Solution

