Mark the correct order of increasing reactivity
A
CH3CONH2<CH3COOC2H5<CH3COCl
B
CH3COOC2H5<CH3COCl<CH3CONH2
C
CH3COCl<CH3CONH2<CH3COOC2H5
D
CH3COOC2H5<CH3CONH2<CH3COCl
Text Solution
The correct Answer is:A
This can be explained on the basis of basicity of leaving group in each case. For acid chlorides, leaving group is Cl− ions and for esters, leaving group is alkoxide. However, in amide, leaving group is NH2 or amine. Out of these three, Cl− ion is weakest base hence acid chlorides are most reactive. But NH2 or amines are strongest bases, hence amides are least reactive
|
Similar Questions
Knowledge Check
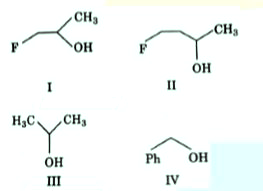
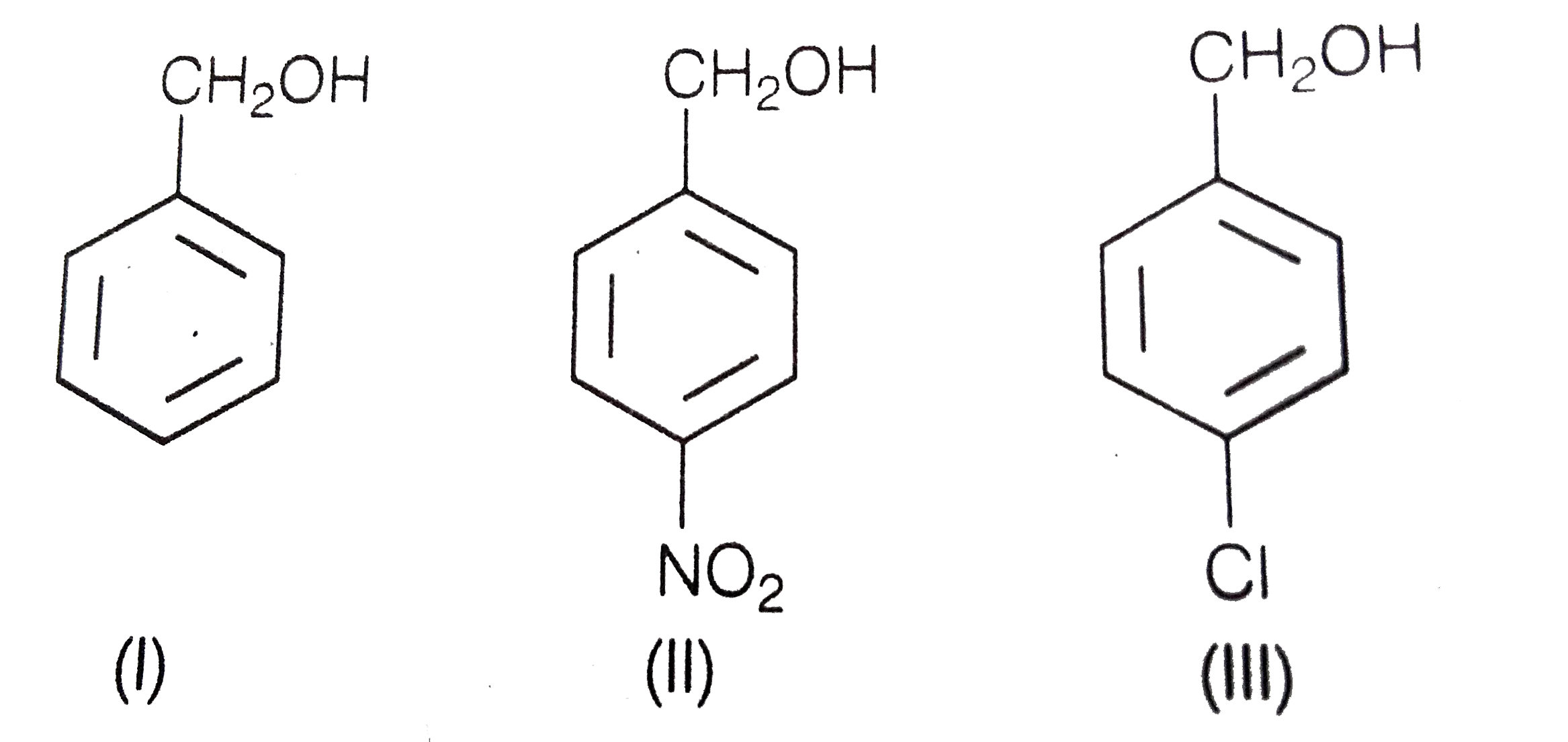
The correct order of increasing reactivity of C−X bond towards nucleophile in the following compounds is:
 .A(II)lt(III)lt(I)lt(IV)B(IV)lt(III)lt(I)lt(II)C(III)lt(II)lt(Ilt(IV)D(I)lt(II)lt(IV)lt(III)
.A(II)lt(III)lt(I)lt(IV)B(IV)lt(III)lt(I)lt(II)C(III)lt(II)lt(Ilt(IV)D(I)lt(II)lt(IV)lt(III)The correct order of increasing reactivity of C-X bond towards nucleophile in the following compounds is :
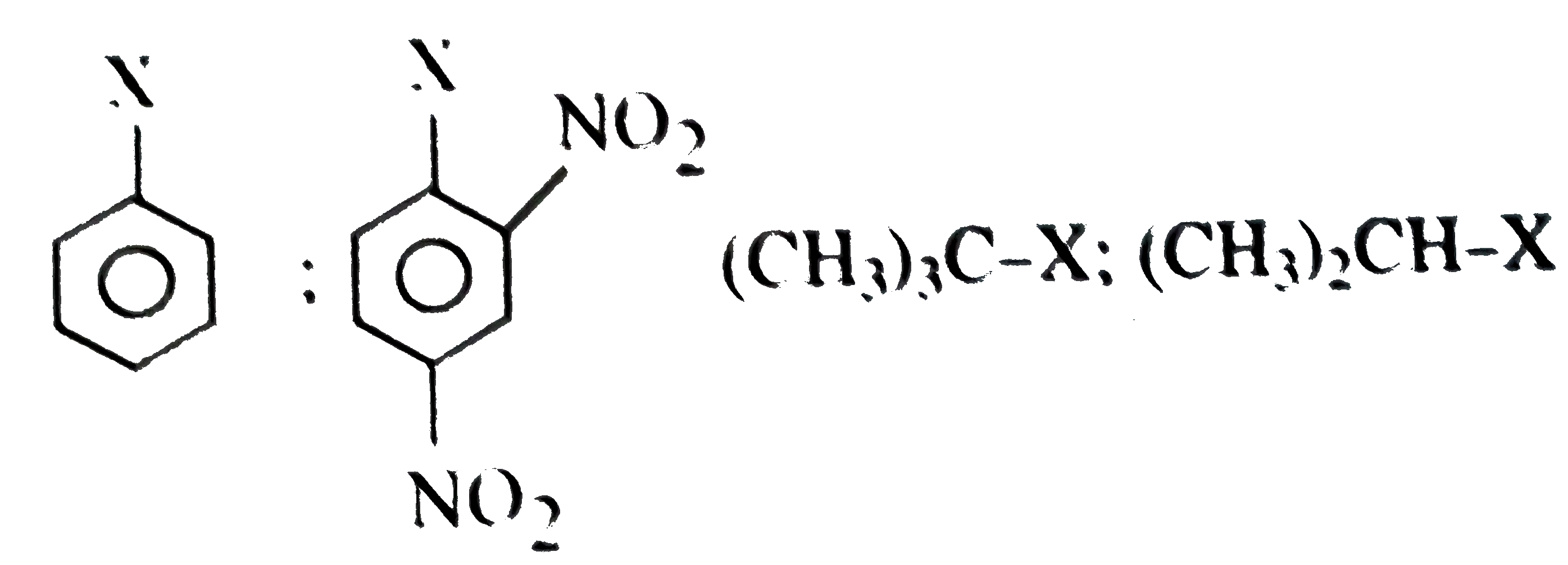 AII lt III lt I lt IVBIV lt III lt I lt IICIII lt II lt I lt IVDI lt II lt IV lt III
AII lt III lt I lt IVBIV lt III lt I lt IICIII lt II lt I lt IVDI lt II lt IV lt IIIThe correct order of increasing reactivity of C –X bond towards nucleophile in the following compound is
AIV<III<I<IIBII<II<I<IVCI<II<IV<IIIDII<III<I<IV
The correct order of increasing reactivity of C-X bond towards nucleophile in the following compounds is
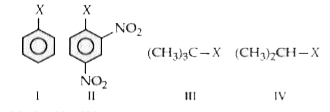
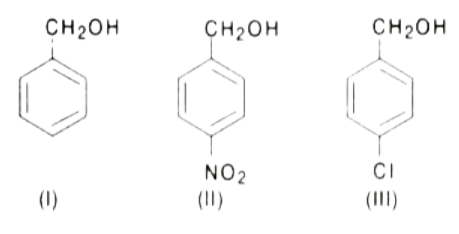
View SolutionWhich of the following represent the correct order of decreasing reactivity?
View SolutionArrange these in correct order of decreasing reactivity
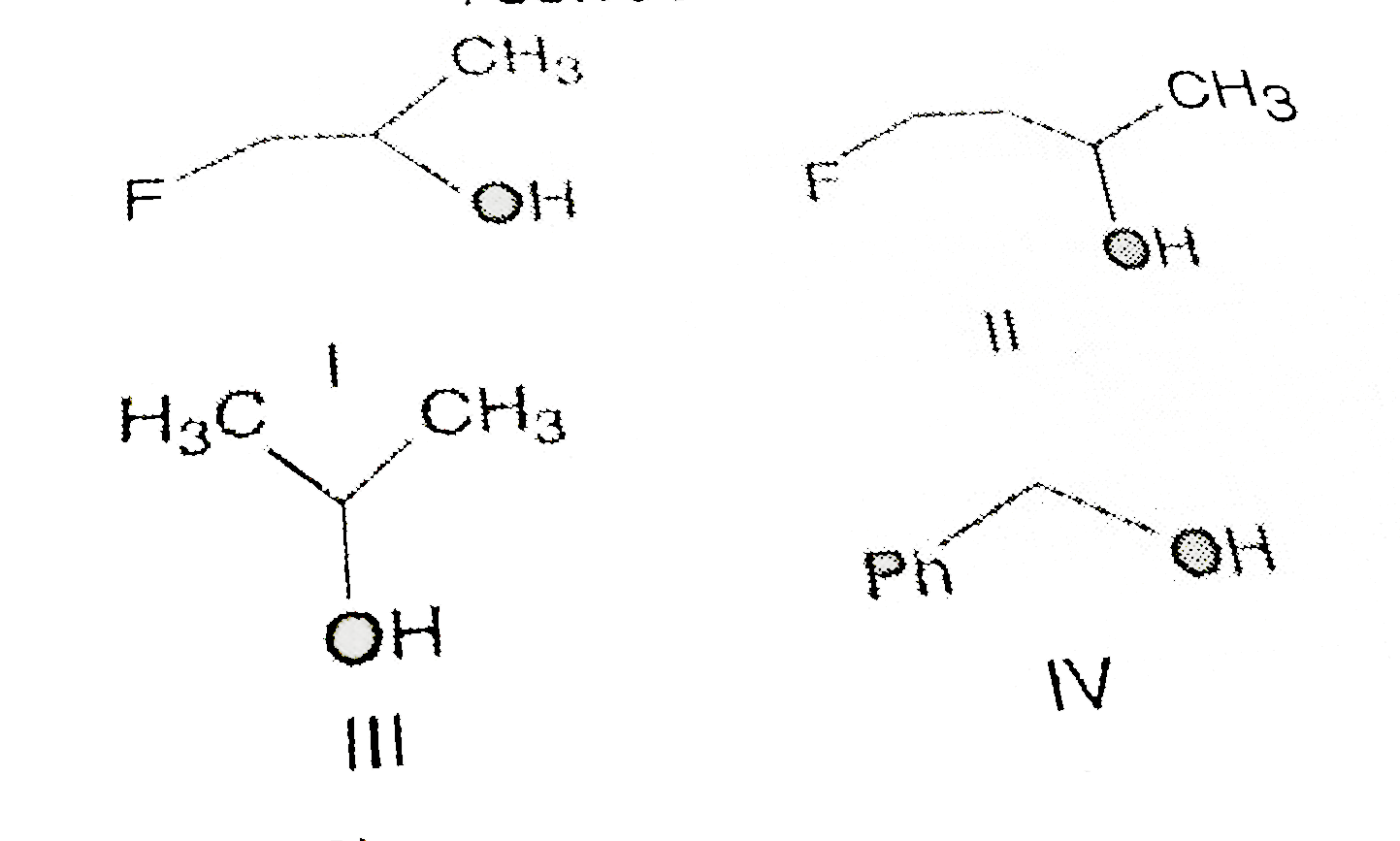
View SolutionArrange these in correct order of decreasing reactivity.
View SolutionMark the correct increasing order of reactivity of the following compounds with HBr/HCl.
View Solution