Reaction of R−CONH2 with Br2/KOH gives R−NH2 as the main product. The intermediate involved in this reaction are
R−O∣∣C−NHBr
R−I↑NH−Br
R−NH−Br
R−O∣∣C−N∣Br−Br
The correct Answer is:A
See Noteworthy points in the Comprehensive Review
Topper's Solved these Questions
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBOOK - DINESH PUBLICATIONCHAPTER - CARBOXYLIC ACIDS AND THEIR DERIVATIVESEXERCISE - Revision question from competitive exams
162 Videos
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBOOK - DINESH PUBLICATIONCHAPTER - CARBOXYLIC ACIDS AND THEIR DERIVATIVESEXERCISE - Selected straight objective type MCQs
43 Videos
- BIOMOLECULESBOOK - DINESH PUBLICATIONCHAPTER - BIOMOLECULESEXERCISE - Reason
1 Video
- CHEMICAL BONDING AND MOLECULAR STRUCTURE BOOK - DINESH PUBLICATIONCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTURE EXERCISE - COMPREHENSION
4 Videos
Similar Questions
Knowledge Check
Reaction of RCONH2 with a mixture of Br2 and KOH gives RNH2 as the main product. The intermediates involved in the reaction are
ARCONHBrBR−NHBrCR−N=C=ODRCONBr2.Reaction of RCONH2 with a mixture of Br2 and KOH gives RNH2 as the main product. The intermediates involved in the reaction are:
AR−OH∣C=N−BrBR−NHBrCR−N=C=OD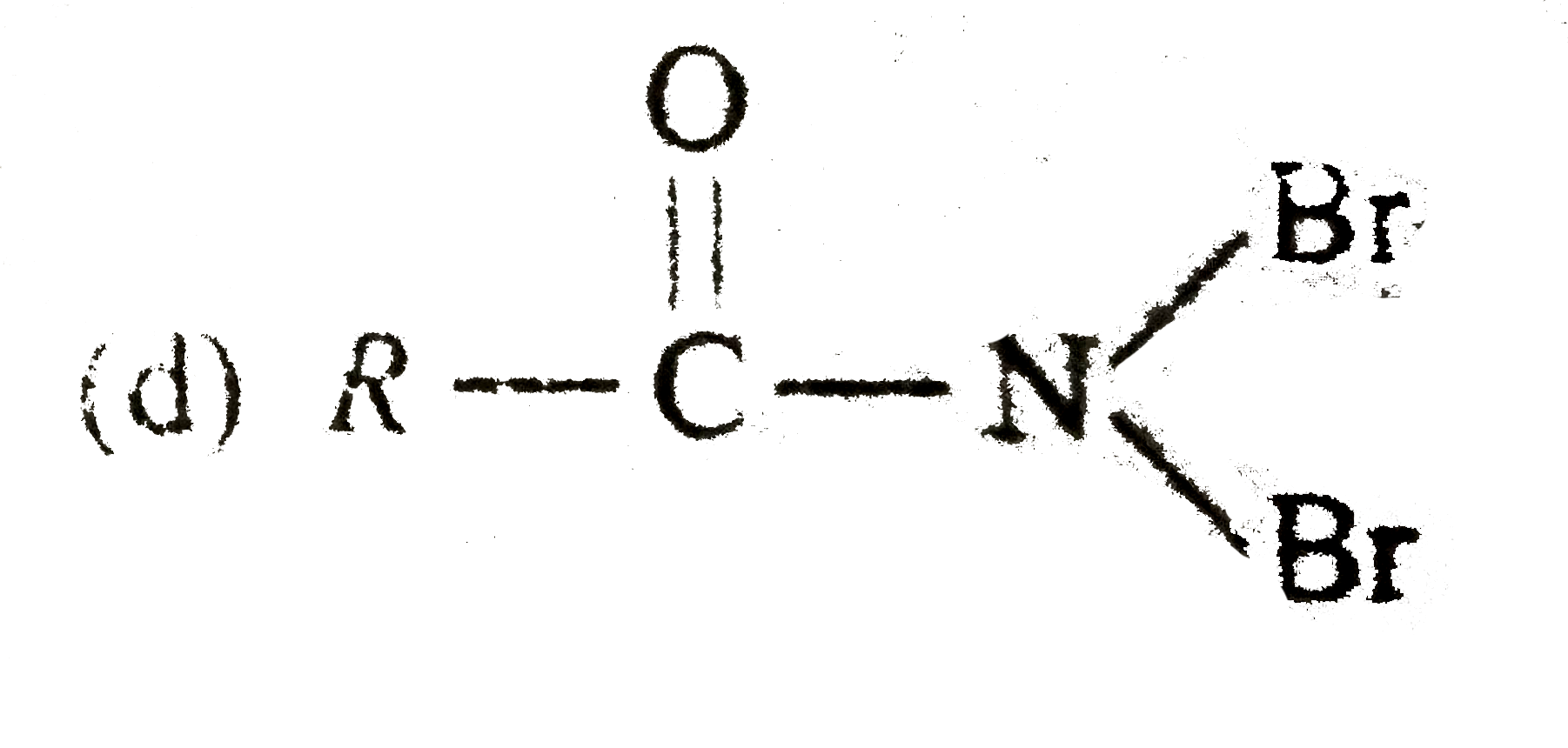
The reaction of RCONH2 with a mixture of Br2 and KOH gives R - NH2 as a product . The intermediates involved in this reaction are .
AR−CONBrBR−N=C=OC−NHBRD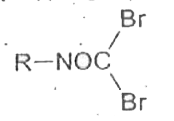
Reaction of R−O∣∣C−NH2 with a mixture of Br2 and KOH gives R−NH2 as the main product . The intemediates (s) involved in this reaction is/are.
View SolutionThe reaction of R−O∣∣C−NH2 With a mixture of Br2 and KOH gives R−NH2 as the main product. The intermediates (s) involved in this reaction is/are
View SolutionThe reation of RCONH2 with a mixture of Br2 and aqueous KOH gives RNH2 as the main product The intermediate (s) involved in this reation is (are) .
View SolutionThe reation of RCONH2 with a mixture of Br2 and aqueous KOH gives RNH2 as the main product The intermediate (s) involved in this reation is (are) .
View SolutionToluene on reaction with CrO3 and Ac2O gives benzaldehyde as the main product. The intermediate compound formed in the reaction is:
View Solution

