Silver acetate +I2CS2−−→
the main prodcut of this reaction is
A
CH3I
B
CHI3
C
CH3COI
D
CH3COOCH3
Text Solution
The correct Answer is:D
CH3COOAg+I2CS2−−→CH3I+AgI+CO2
CH3I+AgOCOH3→CH3COOCH3Methyl acetate
|
Topper's Solved these Questions
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:DINESH PUBLICATIONChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Brain Teasers
Explore 40 Videos
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:DINESH PUBLICATIONChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Unit test
Explore 20 Videos
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:DINESH PUBLICATIONChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Reason Assertion Type MCQs
Explore 10 Videos
- BIOMOLECULESBook:DINESH PUBLICATIONChapter:BIOMOLECULESExercise:Reason
Explore 1 Video
- CHEMICAL BONDING AND MOLECULAR STRUCTURE Book:DINESH PUBLICATIONChapter:CHEMICAL BONDING AND MOLECULAR STRUCTURE Exercise:COMPREHENSION
Explore 4 Videos
Similar Questions
Knowledge Check
Silver Acetate + Br2CS2−−→ Main Product . The major product of this reaction is
ACH3−BrBCH3COICCH3COOHDNone of theseSilver Acetate + Br2CS2−−→ Main Product . The major product of this reaction is
ACH3BrBCH3COICCH3COOHDNone of theseCH3−CH2CH3∣CHCH=CHCH3H3O+−−−→
Major product of the reactionAis an potical isomerBgives white turbidity with with Lucas reagent immediatelyCis dehydrated easilyDAll options are correct.
C6H5NH2+CS2Δ−−−−−→KOH(s)
The end product in the reaction is
View SolutionFor the two step reaction in which both the steps are first order
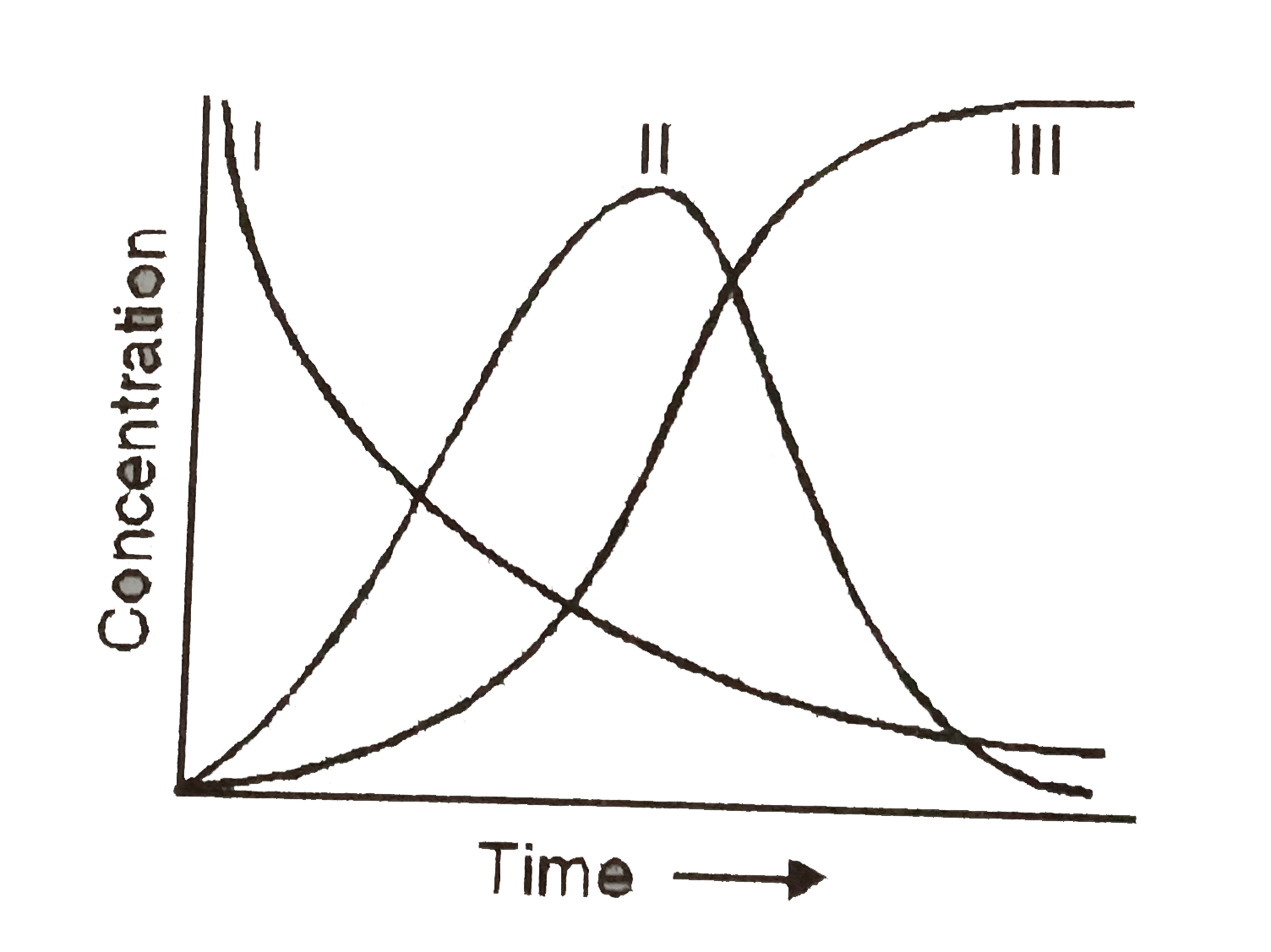
Rk1−−→I
Ik2−−→P
the rate of change of concentration of I is given by the plot
View Solution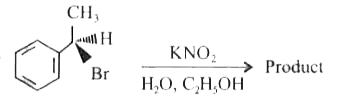 KNO2−−−−−−−−→H2O,C2H5OH Product
KNO2−−−−−−−−→H2O,C2H5OH Product
The main product is
View SolutionCH=CH1%HgSO4−−−−−−→42%H2SO4ANH,H2,Ni−−−−−−→Δ,pressB
The Prodcut B in the above sequence of reaction is
View SolutionIn the following reaction sequence :
R−OHP+I2−−−→R−IAgNO2−−−→RNO2HNO2−−−→ no reaction.
The alcohol is a :
View Solution

