X(i)HCN−−−−−−−−→(ii)dil.H2SO4 2-Hydroxypropinoinc acid
The structure of X is
A
CH3COOH
B
CH2=CH−COOH
C
CH3CHO
D
CH3COCH3
Text Solution
The correct Answer is:C
2-Hydroxpropionic acid is an α− hydroxy acid. This can be prepared from aldehydes or ketones using HCN/H3O+
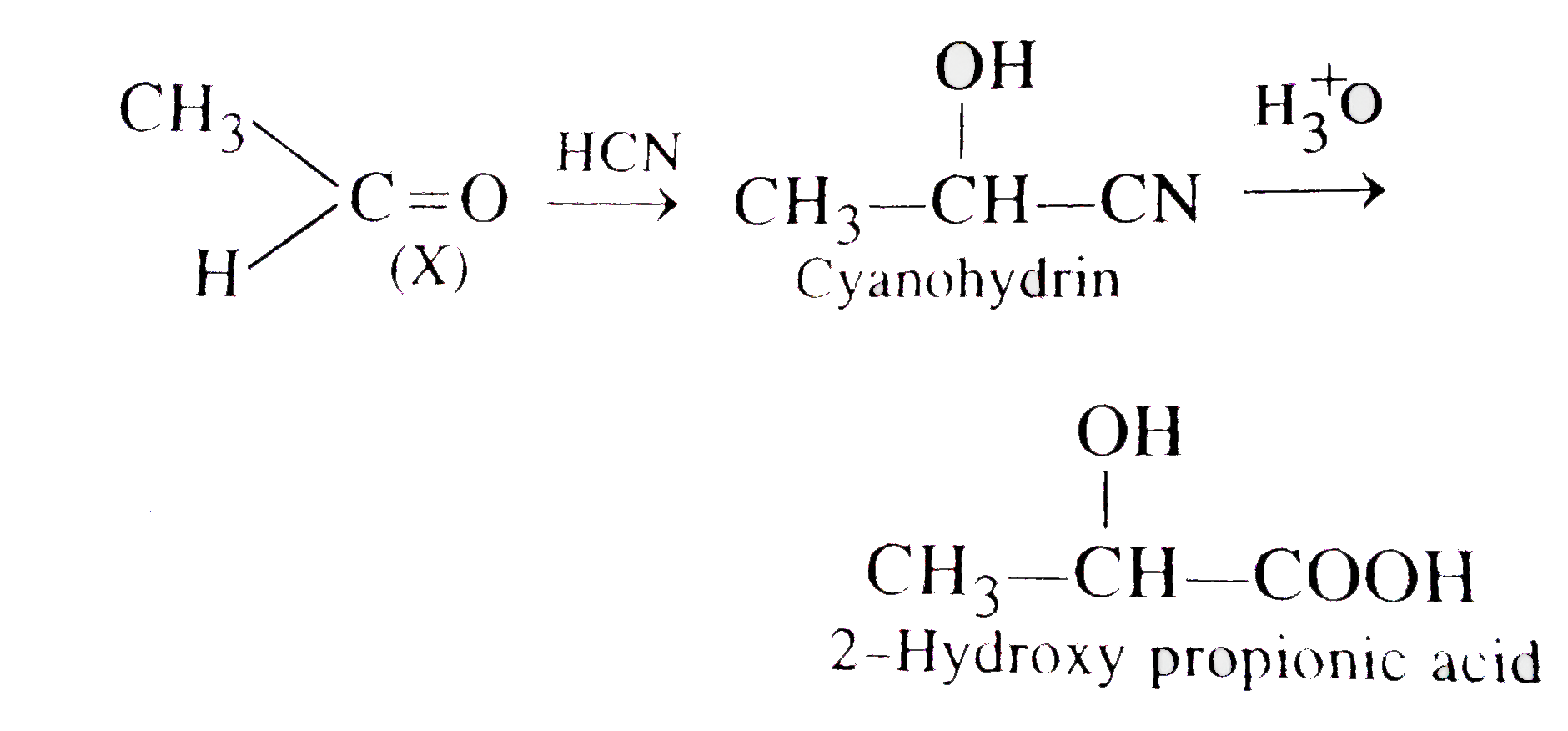
|
Similar Questions
Knowledge Check
X(i)HCN−−−−−−−−→(ii)dil. H2SO4 2-Hydroxypropionic acid
The structure of X isACH3COOHBCH2CH−COOHCCH3CHODCH3COCH3.(White ppt)DNa2CO3←−−−−−−−dil.H2SO4A↓C(White ppt)K2CrO4−−−−−−−−−→(in acetic acid)B(Yellow ppt)
If A is the metallic salt, then the white ppt. of D must be ofAStronsium carbonateBred leadCbarium carbonateDcalcium carbonateH3C−C≡C−N∣CH3−CH3Hg2+/H+−−−−−−→H2O(i)LAH−−−−→(ii)H2OX
Which statement is incorrect for X?AX does not react with Na metalBX react with Heinsberg's reagent to form a product which is insoluble in KOHCX does not evolve N2 on reaction with HNO2DX is soluble in H2SO4
Cumene (i)O2−−−−−−−−−−→(II)H2O,H+(X) and (Y) ,
(X) and (Y)respectively are :
View SolutionProduct 'D' in the following reaction is
CH3CH=CH2(i)B2H6/THF−−−−−−−−−−−−→(ii)H2P2/OH−AK2Cr2O7+−−−−−−−−→dil.H2SO4
BPCl3 −−−−→CH2O −−−→D
View SolutionCH3CHO+HCHODil.NaOH−−−−−−−−→heatAHCN−−−−−→H3OB
The structure of compound B is
View SolutionH−C≡C−HHgSO4−−−→H2SO4(A)(1)NH3+HCN−−−−−−−−−→(2)H3O⊕(B), Product (B) of given reaction is :
View SolutionCumene (i)O2−−−−−−−−−−→(II)H2O,H+(X) and (Y) ,
(X) and (Y)respectively are :
View Solution

