Given three acids
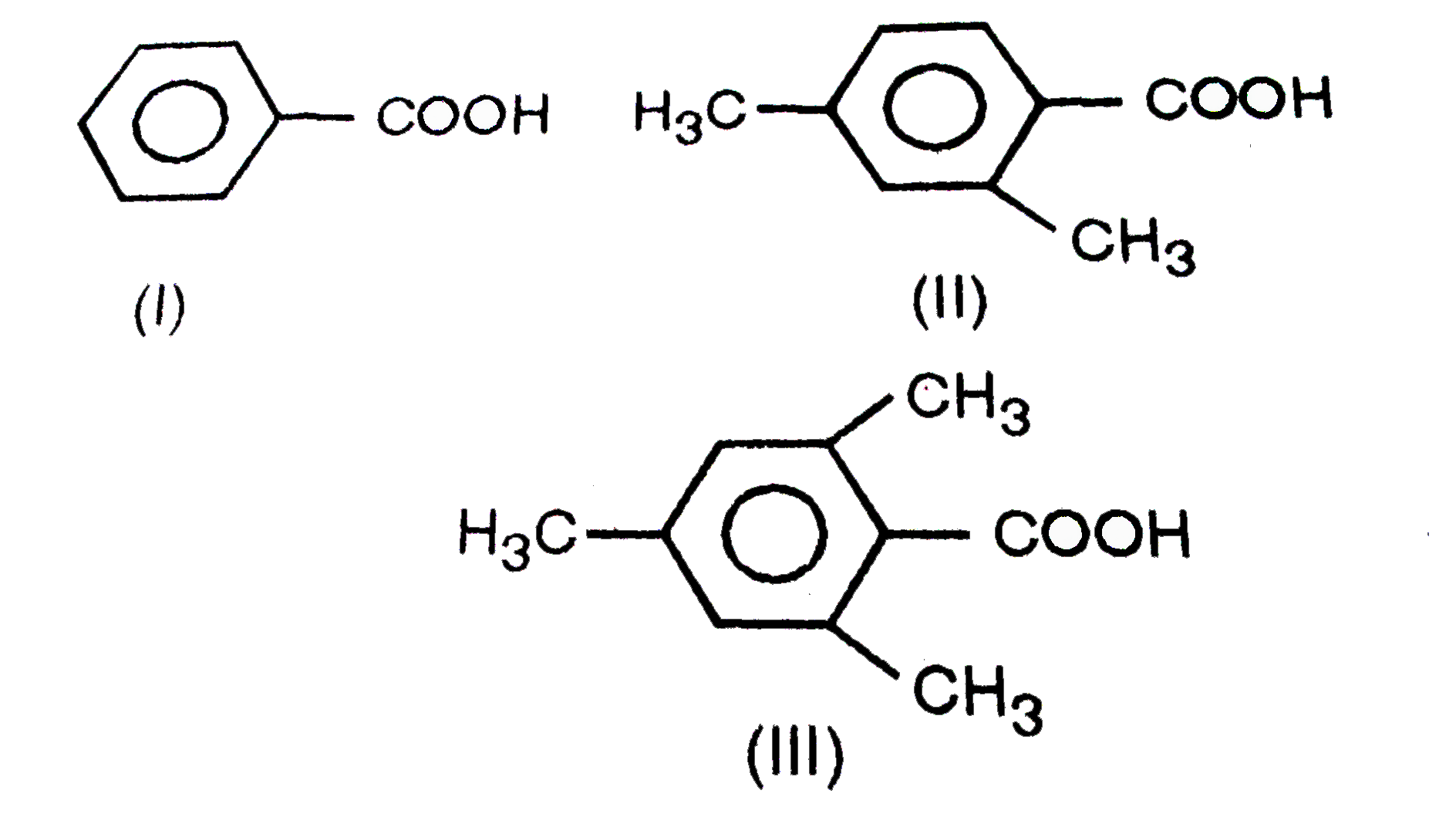
The order of ease of acid catalysed esterification is
A
I>III>II
B
I>II>III
C
III>I>II
D
II>III>I
Text Solution
The correct Answer is:B
Larger the number of electron releasing alkyl groups at α−carbon, less the susceptibility of the acid to the nucleophilic attack of alcohol. Thus the order of esterification is I>II>III
|
Similar Questions
Acid-catalysed estrification of a carboxylic acid is shown below.
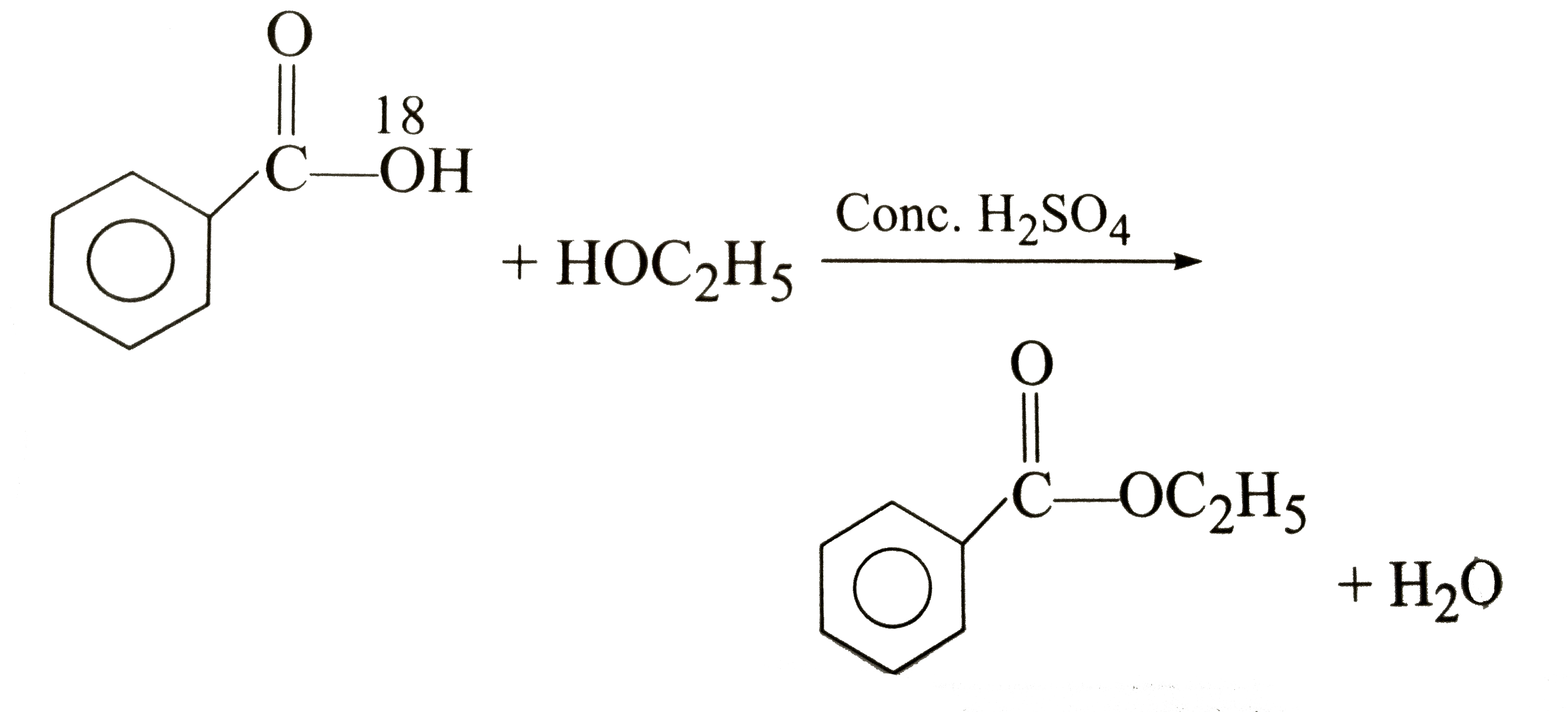
(i) What special name is given to this esterification reaction?
(ii) Trace the position of O18 of benzoic acid in the products formed.
(iii) Acid catalysed esterification is a substitution at the carbonyl carbon or at the oxygen of OH. Explain
View SolutionAcid Catalysed Reaction With Water
View Solution
Knowledge Check
The correct decreasing order of ease of acid catalysed estrification is :
ACH3[CH2]3OH>CH3CH2C(OH)CH3>(CH)3COHBCH3C(OH)C2H5>CH3[CH2]3OH>(CH3)3COHC(CH3)3COH>CH3CH2C(OH)CH3>CH3[CH2]3OHDNone of these is correct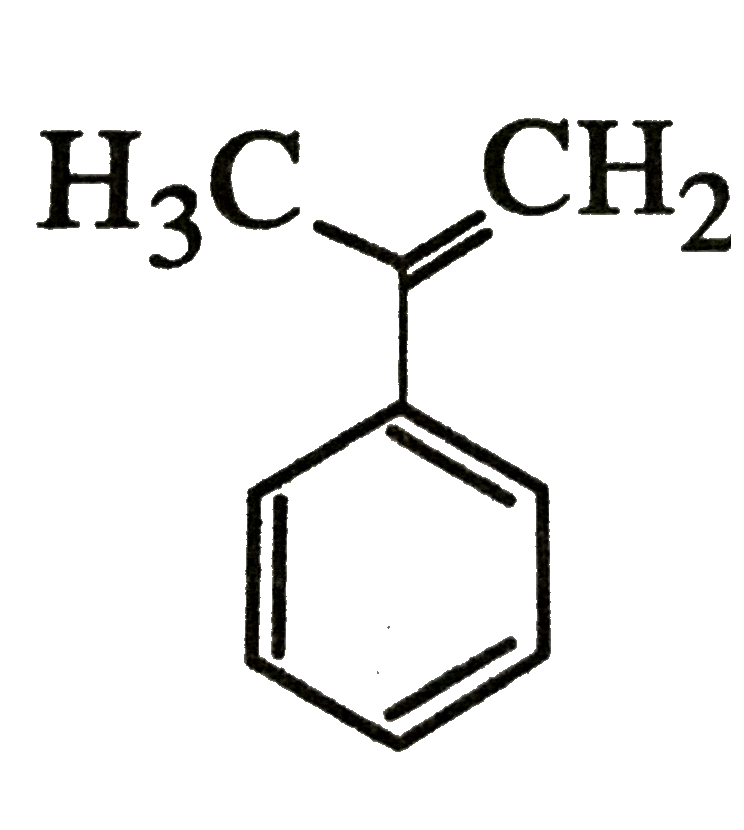 on acid catalysed hydration givesA
on acid catalysed hydration givesA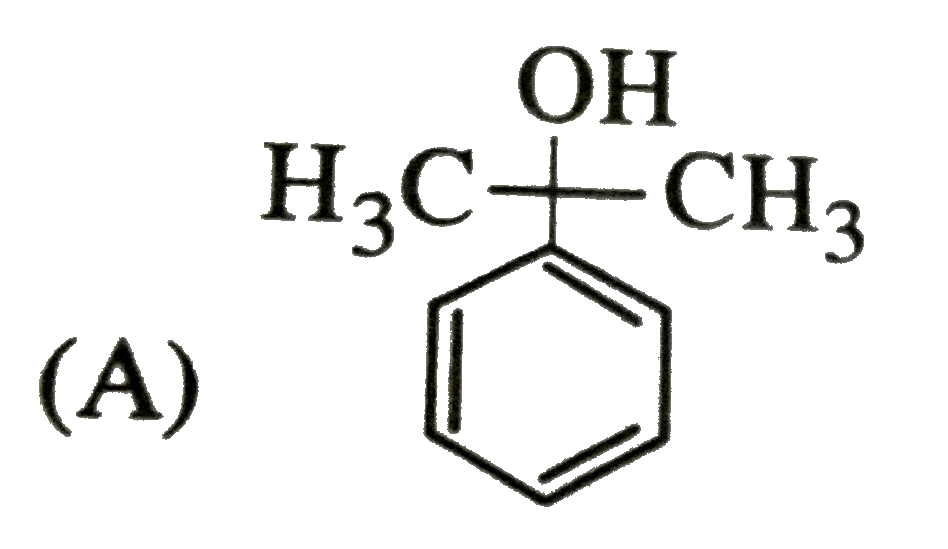 B
B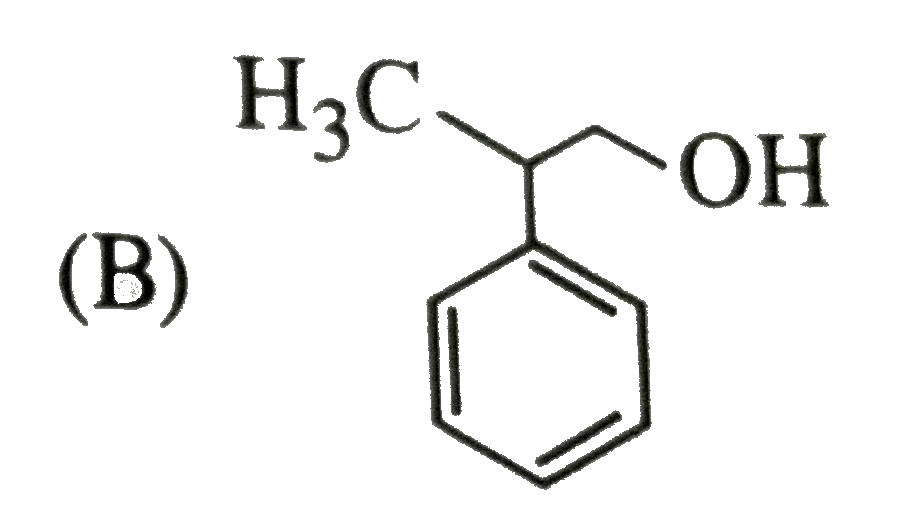 C
C DNone of these
DNone of theseThe order of a reaction for an esterification process is:
AZeroBFirstCSecondDPseudo first order
The order of a reaction for an esterification process is:
View SolutionThe correct order of acid catalysed dehydration of alcohols is:
View SolutionThe relative order of rate of esterification of acids is
View SolutionWhich of the following intermediates are involved in the acid catalyzed esterification of carboxylic acid?
View Solution
The order of decreasing acidity of these acidic sites is :
View Solution

