In which of the following the C−O bonds are equal length
A
CH3COO−
B
CH3COOH
C
CH3COOH3
D
In all
Text Solution
The correct Answer is:A
Acetate ion has two equivalent resonating structures, others have non-equivalent resonating structure
|
Similar Questions
Knowledge Check
Which of the following has longest C-O bond
A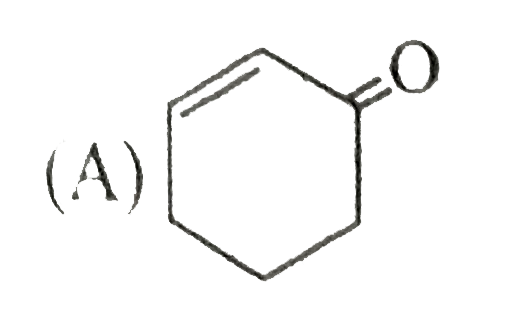 B
B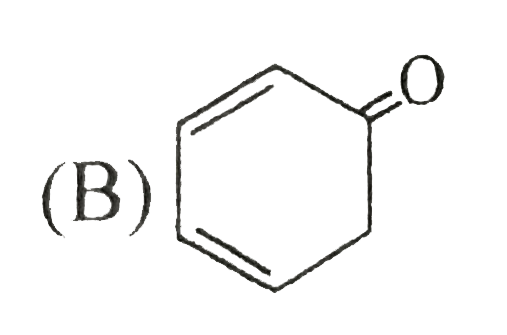 C
C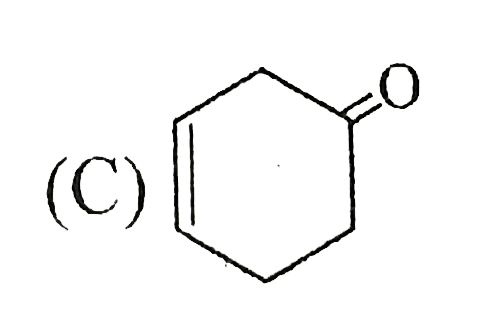 D
D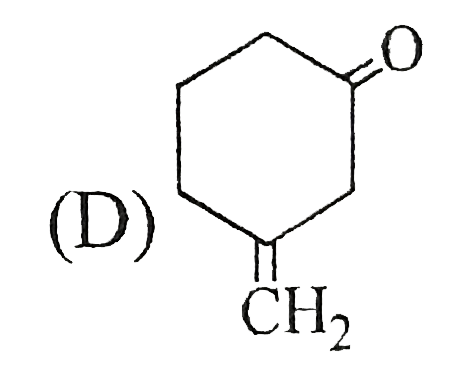
In which of the following the carbon-oxygen bonds are of equal lengths?
ACH3COO−BCH3COOHCCH3COOC2H5DAll of theseWhich of the following has longest C-O bond?
A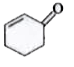 B
B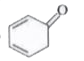 C
C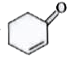 D
D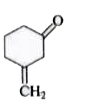
Which of the following has longest C-O bond?
View SolutionWhich of the following has longest C–O bond:
View SolutionIn which of the following metal carbonyls, the C-O bond length is minimum ?
View SolutionIn which of the following molecule all bond length are equal :-
View SolutionIn which of the following molecule, the bond lengths are not equal
View Solution

