Fluorine is more electronegative than either boron or phosphorus. What conclusion can be drawn from the fact that BF3 has no dipole moment nut PF3 does ?
BF3 is not spherically symmetrical but PF3 is
BF3 molecule must be linear
The atomic radius of P is larger than that of B
The BF3 molecule must be planar triangular
The correct Answer is:D
The B-F bond is polar, yet BF3 is non-polar because BF3 has a planar triangular structure.
Topper's Solved these Questions
Similar Questions
Although F is more electronegative than H , then
resultant dipole moment of NH3 is much moe than
that of NF3 . It can be explained as
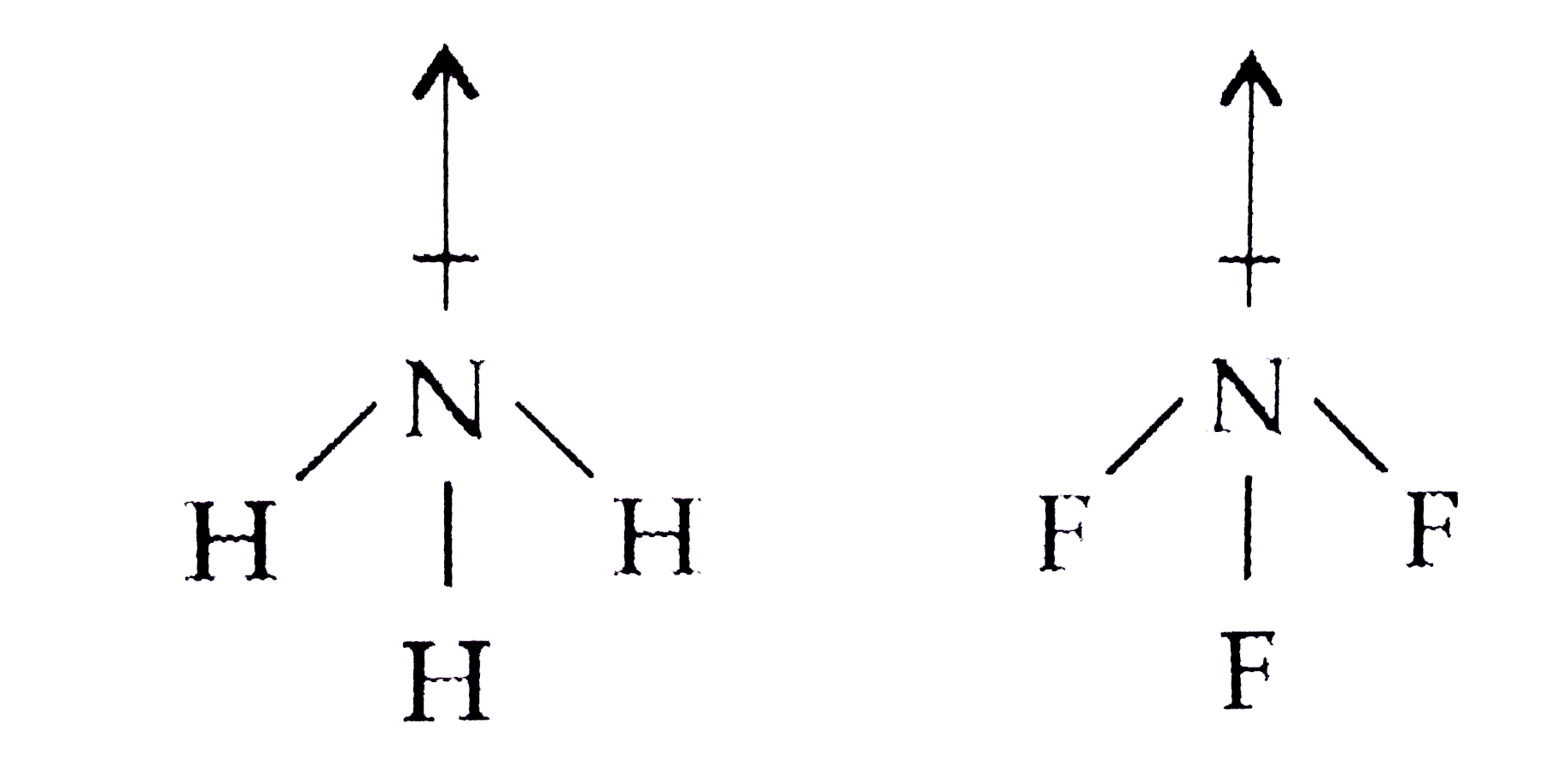
View SolutionAccount for the following observations
(a) AlCl3 is a Lewis acid
(b) Though fluorine is more electronegative than chlorine yet BF3 is a weaker Lewis acid than CI3
(c) PbO2 is a stronger oxidising agent than SnO2
(d) The +1 oxidation state of thallium is more stable than its +3 state.
View Solution
Knowledge Check
Fluorine is more electronegative than either boron or phosphorous. What conclusion can be drawn from the fact that BF3 has no dipole moment but PF3 does
ABF3 is not spherically symmetrical but PF3 is symmetricalBBF3 molecule must be linearCThe atomic radius of F is larger than the atomic radius of BDThe BF3 molecule must be planar triangularCH3Cl has more dipole moment than CH3F because :
Aelectron affinity of chlorine is greater than that of fluorineBthe charge separation is larger in CH3Cl compared to CH3FCthe repulsion between the bond pairs and non-bonded pairs of electrons is greater in CH3Cl than CH3FDchlorine has higher electronegativity than fluorineIn each of the following questions two statements are given followed by two conclusions I and II. You have to consider the two statements to be true, even if they seem to be at variance from commonly known facts. You are to decide which of the given conclusions can definitely be drawn from the given statements. Indicate your answer.
Statements :
(I) Most No : 64 buses go to my office.
(II) This is a No: 64 bus.
Conclusions :
(I) This bus goes to my office.
(II) This bus does not go to my office.Aa) I and II followBb) I does not follow and II followsCc) II does not follow and I followDd) Neither follows
Account for the following observations
(a) AlCl3 is a Lewis acid
(b) Though fluorine is more electronegative than chlorine yet BF3 is a weaker Lewis acid than CI3
(c) PbO2 is a stronger oxidising agent than SnO2
(d) The +1 oxidation state of thallium is more stable than its +3 state.
View SolutionAccount for the following observation:
(i) AlCl3 is Lewis acid
(ii) Though fluorine is more electronegative than chlorine yet BF3 is a weaker Lewis acid than BCl3
(iii) PbO2 is a stronger oxidising agent thatn SnO2
(iv) The +1 oxidation state of thallium is more stable than its +3state.
View SolutionAlthough fluorine is more electronegative than chlorine but chlorine can be converted into chloride ion more easily as compared to fluoride ion from fluorine . Explain.
View SolutionAs N−F bond is more polar than N−H bond, NF3 molecule has higher dipole moment than NH3.
View SolutionAlthough fluorine is much more electronegative than hydrogen yet the dipole moment of NF3 (0.24 D) is much lower than that of NH3 (1.46 D). Explain.
View Solution

