Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of the following has the highest dipole moment?
A
CO2
B
HI
C
H2O
D
SO2
Video Solution
Text Solution
The correct Answer is:C
c) CO2 being symmetrical has zero dipole moment.
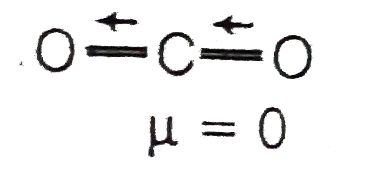
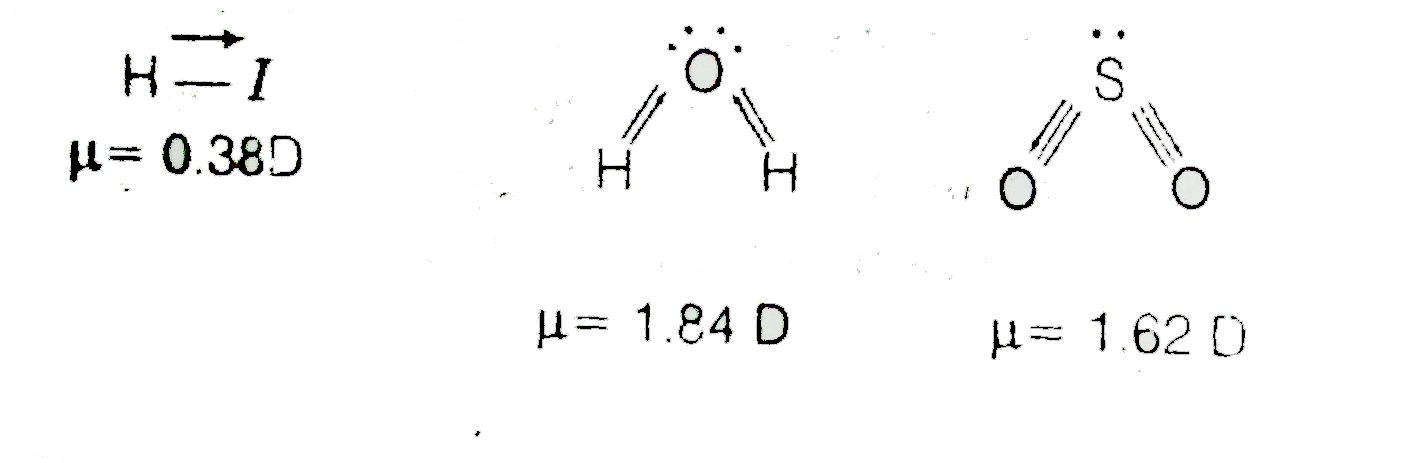
Among HI, SO2 and H2O dipole moment is highest for H2O as in it the central atom contains 2 lone pairs.
|
Similar Questions
Knowledge Check
Which one of the following has the highest dipole moment ?
AAsH3BSbH3CPH3DNH3Which one of the following has the highest dipole moment ?
ANH3BPH3CSbH3DAsH3Which of the following has highest dipole moment?
ANH3BPH3CAsH3DSbH3
Which of the following has highest dipole moment:
View SolutionWhich has the highest dipole moment?
View SolutionWhich of the following species has the highest dipole moment?
View SolutionWhich of the following hydries has the highest dipole moments?
View SolutionWhich of the following molecule has highest dipole moment?
View Solution

