Which of the following compound will give positive Tollen’s test
CH3CHO
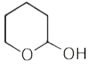
CH3CH2CH2CH2CH∣OH−OCH3
The correct Answer is:A, B, C
To determine which of the given compounds will give a positive Tollen’s test, we need to understand the nature of the Tollen's reagent and the conditions under which a positive test occurs.
1. Understanding Tollen's Test:
- Tollen's reagent is a solution of silver nitrate (AgNO3) in ammonia (NH3). It acts as a mild oxidizing agent.
- It is specifically used to test for aldehydes. When an aldehyde is present, it gets oxidized to a carboxylic acid, and silver ions (Ag+) in the reagent are reduced to metallic silver (Ag), which forms a silver mirror on the test tube.
2. Identifying Aldehydes:
- Only aldehydes can give a positive Tollen’s test. Ketones typically do not react with Tollen's reagent under mild conditions.
3. Analyzing Each Compound:
- Compound 1: If this compound is an aldehyde (RCHO), it will give a positive Tollen’s test.
- Compound 2: If this compound can be converted to an aldehyde under basic conditions (for example, through tautomerization), it will also give a positive test.
- Compound 3: Similar to Compound 2, if this compound can form an aldehyde, it will give a positive test.
- Compound 4: If this compound does not have the ability to form an aldehyde (for example, if it is a ketone or lacks a reactive hydrogen), it will not give a positive test.
4. Conclusion:
- After analyzing all the compounds, we conclude that Compounds 1, 2, and 3 will give a positive Tollen’s test, while Compound 4 will not.
Summary:
- Positive Tollen’s Test: Compounds 1, 2, and 3 (if they contain aldehyde groups or can be converted to aldehydes).
- Negative Tollen’s Test: Compound 4 (if it does not contain an aldehyde group).
- Tollen's reagent is a solution of silver nitrate (AgNO3) in ammonia (NH3). It acts as a mild oxidizing agent.
- It is specifically used to test for aldehydes. When an aldehyde is present, it gets oxidized to a carboxylic acid, and silver ions (Ag+) in the reagent are reduced to metallic silver (Ag), which forms a silver mirror on the test tube.
- Only aldehydes can give a positive Tollen’s test. Ketones typically do not react with Tollen's reagent under mild conditions.
- Compound 1: If this compound is an aldehyde (RCHO), it will give a positive Tollen’s test.
- Compound 2: If this compound can be converted to an aldehyde under basic conditions (for example, through tautomerization), it will also give a positive test.
- Compound 3: Similar to Compound 2, if this compound can form an aldehyde, it will give a positive test.
- Compound 4: If this compound does not have the ability to form an aldehyde (for example, if it is a ketone or lacks a reactive hydrogen), it will not give a positive test.
- After analyzing all the compounds, we conclude that Compounds 1, 2, and 3 will give a positive Tollen’s test, while Compound 4 will not.
- Positive Tollen’s Test: Compounds 1, 2, and 3 (if they contain aldehyde groups or can be converted to aldehydes).
- Negative Tollen’s Test: Compound 4 (if it does not contain an aldehyde group).
Topper's Solved these Questions
- CARBONL COMPOUNDSBook:MOTIONChapter:CARBONL COMPOUNDSExercise:EXERCISE - 3
Explore 10 Videos
- CARBONL COMPOUNDSBook:MOTIONChapter:CARBONL COMPOUNDSExercise:EXERCISE - 4 LEVEL -I
Explore 6 Videos
- CARBONL COMPOUNDSBook:MOTIONChapter:CARBONL COMPOUNDSExercise:EXERCISE - 2 (LEVEL -I)
Explore 46 Videos
- BIOMOLECULES & POLYMERSBook:MOTIONChapter:BIOMOLECULES & POLYMERSExercise:EXERCISE - 4 (LEVEL II)
Explore 17 Videos
- CARBOXYLIC ACIDBook:MOTIONChapter:CARBOXYLIC ACIDExercise:Exercise - 4 (LEVEL - 2 )
Explore 22 Videos
Similar Questions
Predict the product of the reaction of propanal with each of the following
(a) Lithium aluminum hydride
(b) Sodium borohydride
(c) Hydrogen(nickel catalyst)
(d) Methylmagnesium iodide, followed by dilute acid.
(e) Sodium acetylide, followed by dilute acid
(f) Phenyllithium, followed by dilute acid
(g) Methanol containing dissolved hydrogen chloride
(h) Ethylene glycol, p-toluenesulfonic acid, benzene
(i) Aniline (C6H5NH2)
(j) Dimethylamine, p-toluenesulfonic acid, benzene
(k) Hyderoxylamine
(l) Hydrazine
(m) Product of part(l) heated in triethylene glycol with sodium hydroxide
(n) p- Nitrophenylhydrazine
(o) Semicarbazide
(p) Ethylidenetriphenylphosphorane [(C6H5)3+P–¯¯¯..CHCH3] (q) Sodium cyanide with addition of sulfuric acid
(r) Chromic acid
View SolutionThe best reagent to convert pent-3-en-2-ol into pent-3-en-2-one is
View SolutionGem dilhalide on hydrolysis gives:
View SolutionWhich of the following pairs give positive Tollen's test ?
View SolutionThe IUPAC name of C6H5COCl is
View SolutionWhich of the following compounds give positive Tollen's test ?
View SolutionWhich of the following compounds will give a positive iodoform test ?
View SolutionWhich of the following compounds will give positive idodoforms test?
View SolutionWhich of the following compounds will give a positive iodoform test ?
View SolutionWhich of the following compounds will give a positive iodoform test ?
View SolutionWhich of the following compound will give positive iodoform test ?
View SolutionWhich of the following compound will give positive iodoform test ?
View SolutionWhich of the following compound will give positive iodoform test ?
View Solutionनिम्नलिखित यौगिकों में से कौन धनात्मक आयोडोफॉर्म परीक्षण देता
View SolutionWhich of the following compound gives a positive iodoform test:
View Solution
