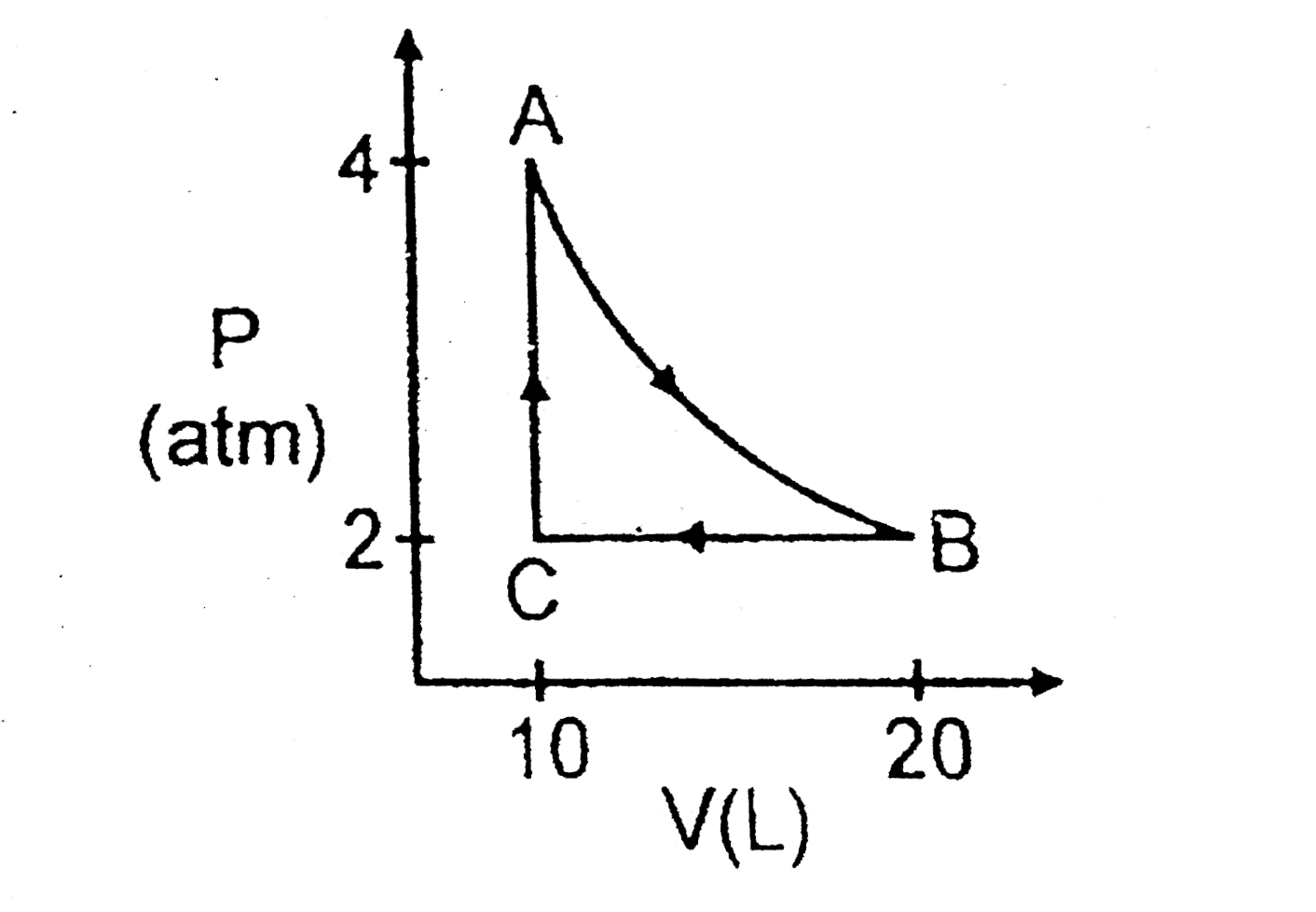
Two moles of an ideal gas undergoes the following process. Given that (∂P∂T)Vis x×10+y, then calculate the value of (x + Y)
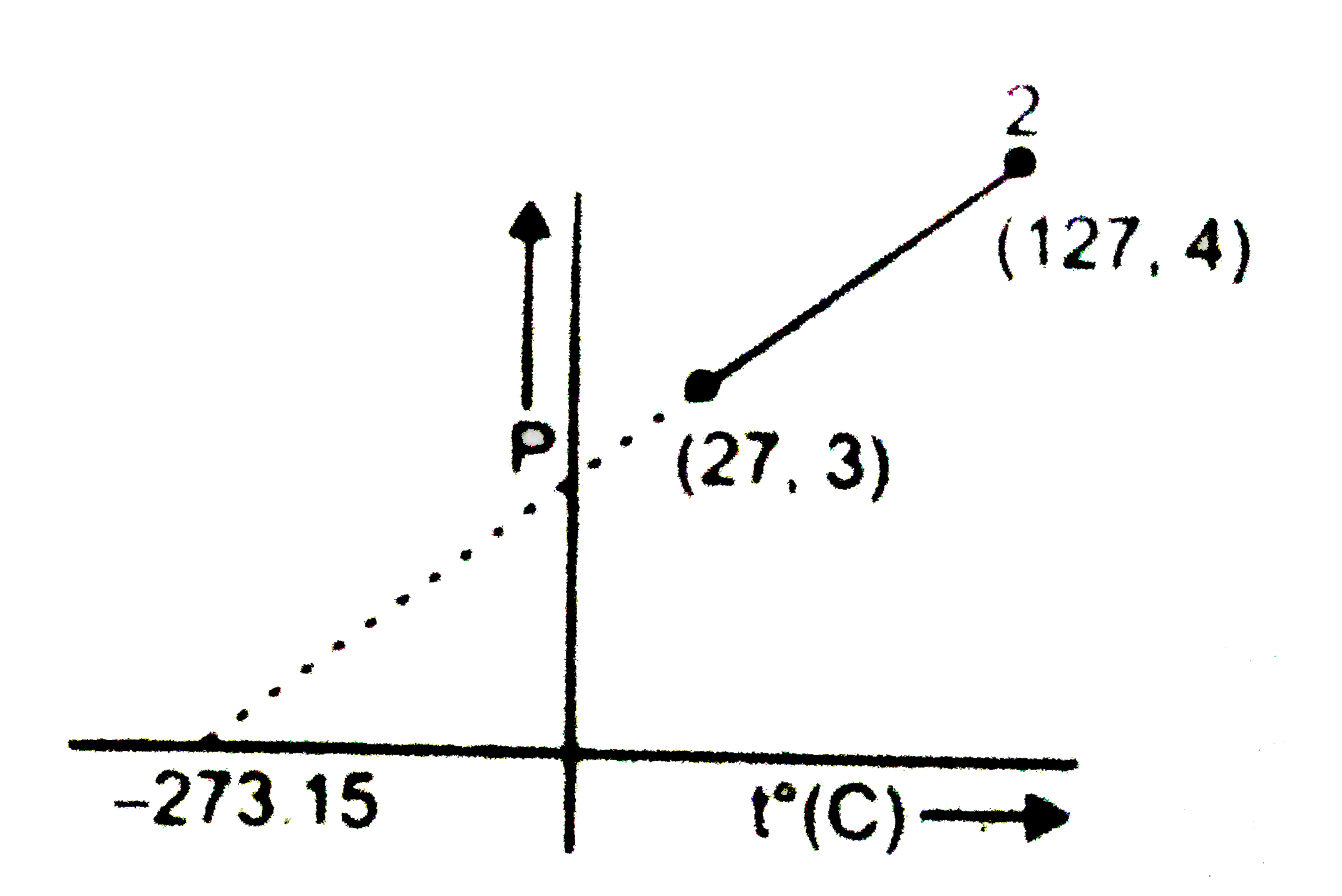
The correct Answer is:3
T−300=4−3100(P−3)
P−300=P100−3100
Multiply by 100
T×100−3×104=P−3
P=3−3×104+100T
dPdT=0+100=1×102
x+y=3
Similar Questions
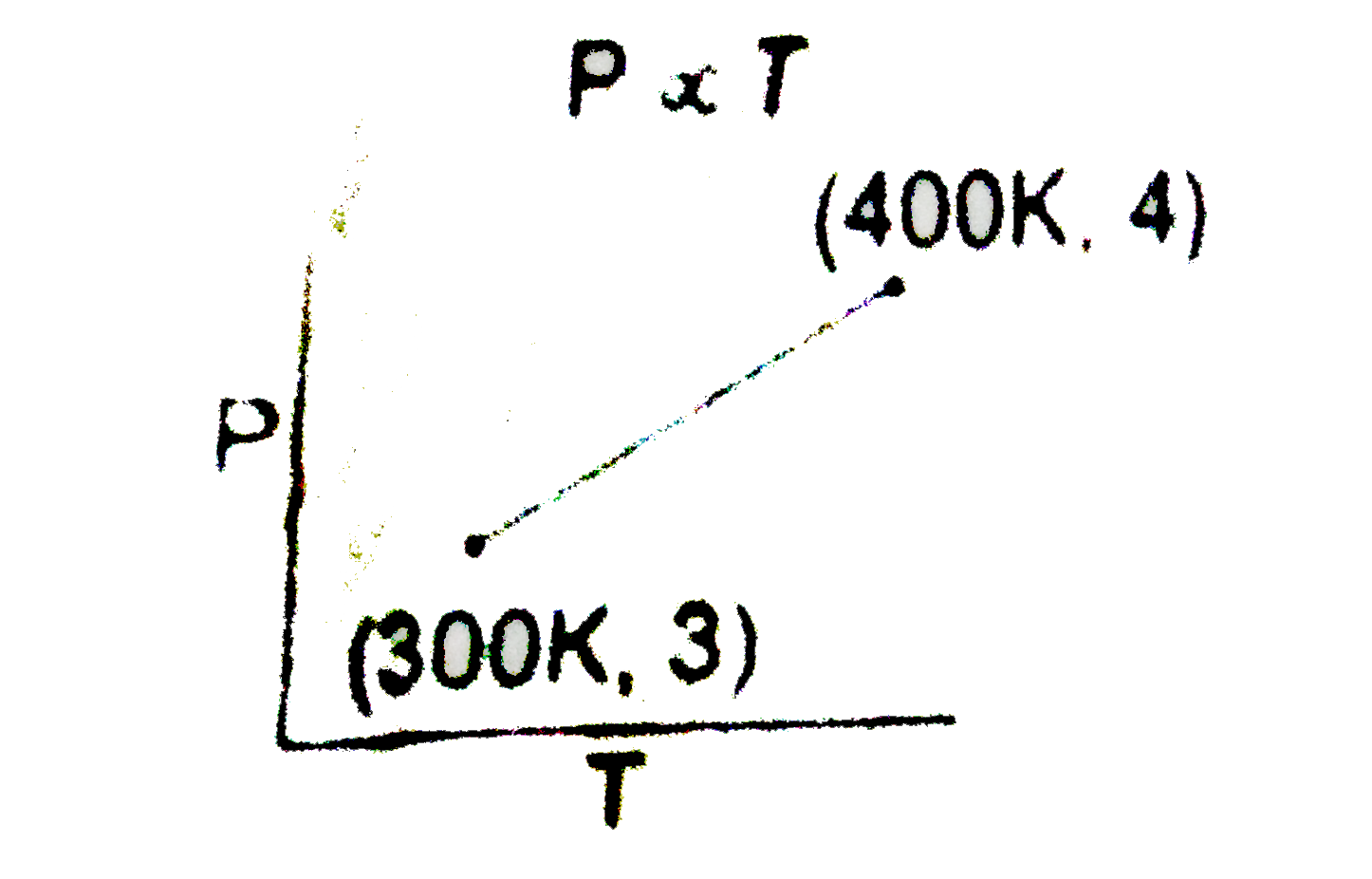
Two moles of an ideal gas undergoes the process depicted below. Given that (δPδT)v is x×10−y, then calculate the value of (y-x).
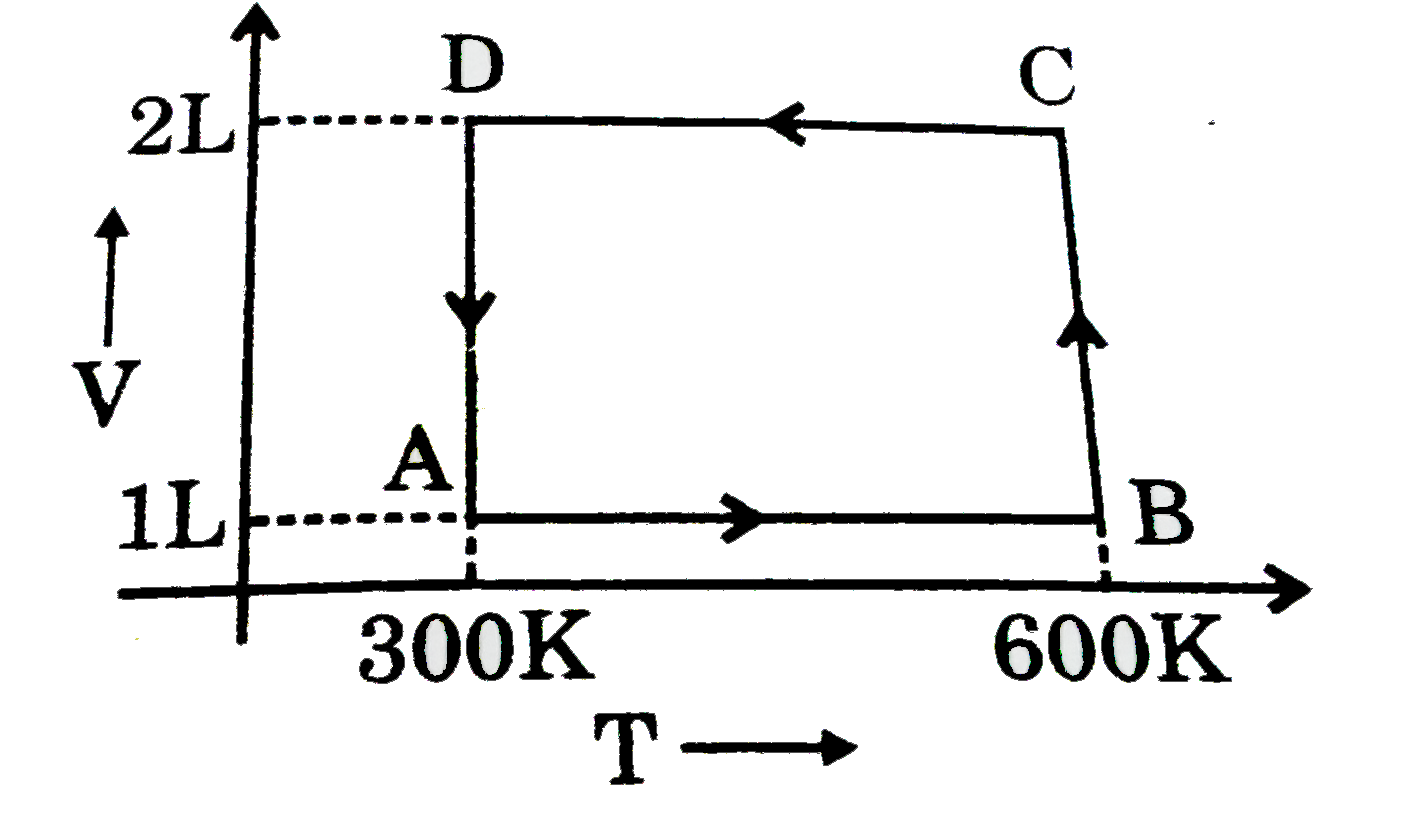
View SolutionOne mole of an ideal monoatomic gas undergoes the following cyclic process. Calculate the magnitude of work (in cal) in a cycle. (Use: ln 2 = 0.7)
View Solution
Knowledge Check
A monoatomic ideal gas undergoes a process ABC. The heat given to the gas is
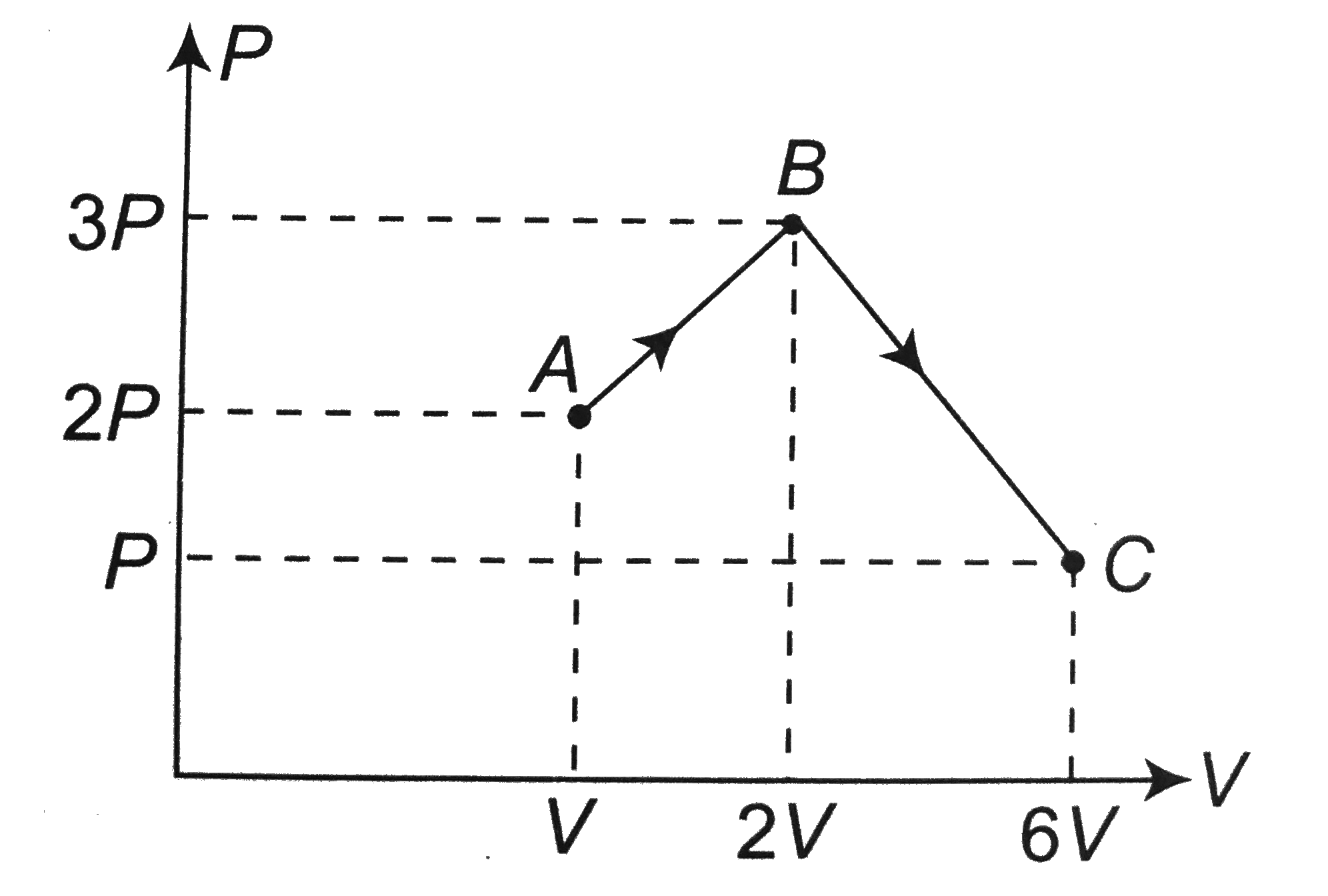 A7.5PVB12.5PVC16.5PVD20.5PV
A7.5PVB12.5PVC16.5PVD20.5PVOne mole Of an ideal triatomic gas undergoes compression process adiabatically . Then:
ATemperature change of gas will be more if process is carried out reversiblyBWork involved during the process will be more if process is carreid out reversiblyCChange in internal energy of gas will be less if process is carreid out reversiblyDChange in enthalphy of gas will be less if process is carried out reversiblyOne mole of a monoatomic ideal gas undergoes a thermodynamic process such thatV3T2= constant. Then,
Awork done by the gas is 200R when temperature of the gas is raised by 300 KBthe specific heat of the gas in the process is 136RCwork done by the gas is 300R when temperature of the gas maised by 600 KDthe specific heat of the gas in the process is 214 R
One mole of a monoatomic ideal gas undergoes the process A→B in the given P-V diagram. What is the specific heat for this process?
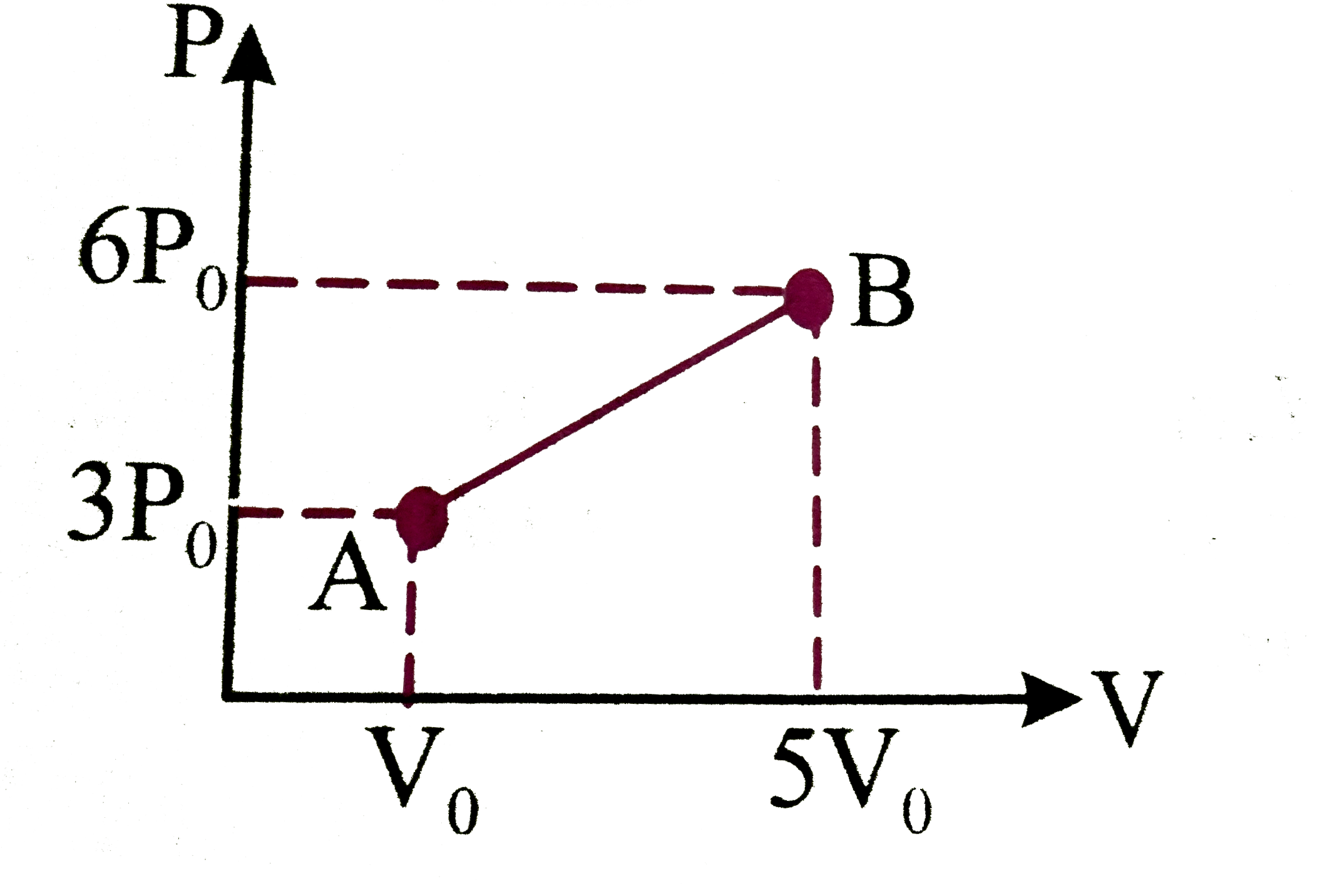
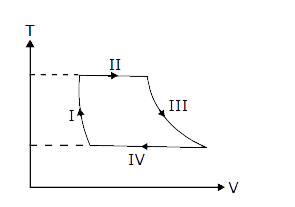
View SolutionOne mole of a monatomic ideal gas undergoes a cyclic process as shown in the figure (where V is the volume and T is the temperature). Which of the statements below is (are) true ?
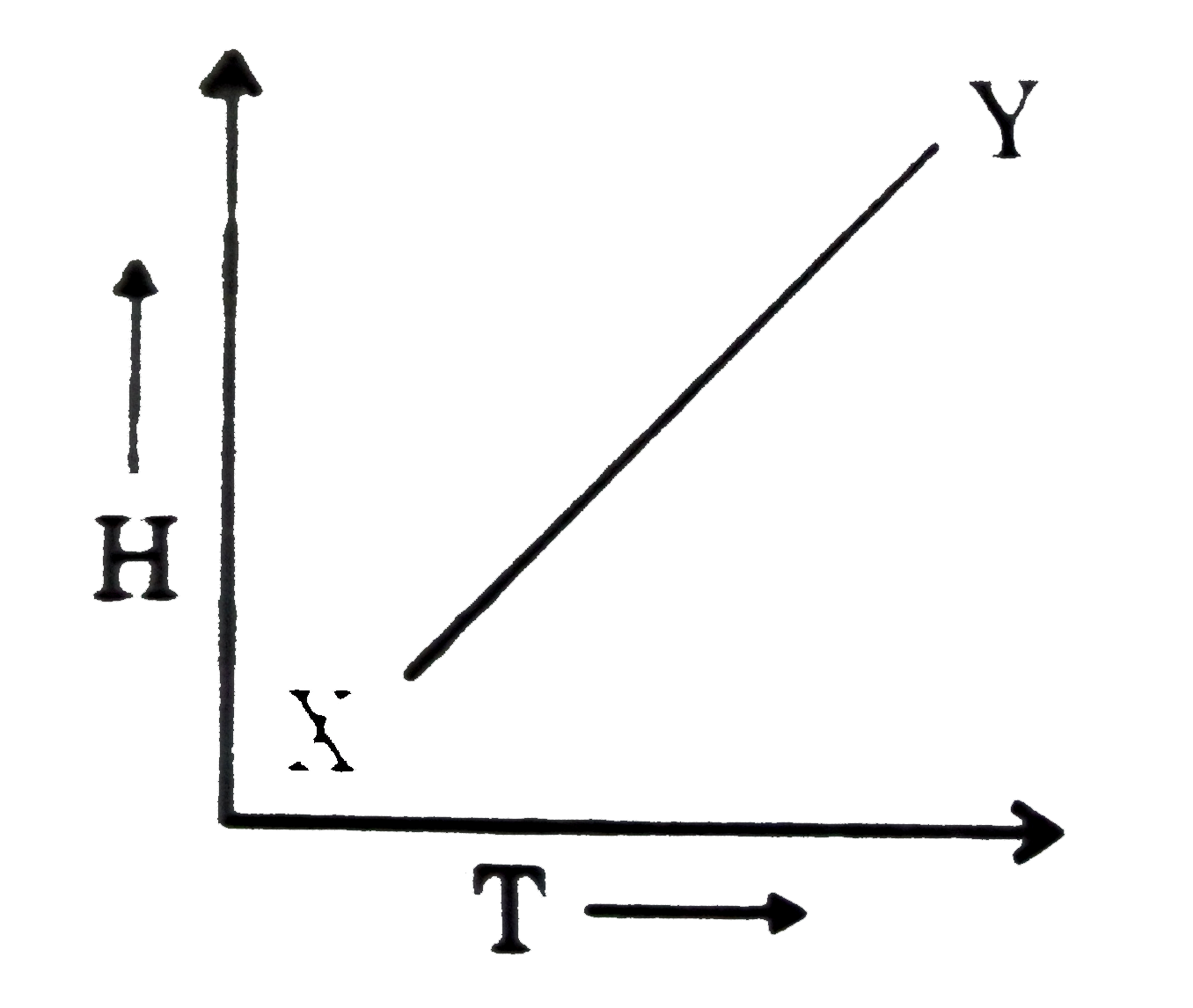
View SolutionThe process in which an ideal gas undergoes change from X to Y as shown in the figure diagram is :
View Solution1 mol of an ideal gas undergoes different thermodynamical process in P−V diragram given below :
What will be the value of ΔH and ΔE for overall process ?
View SolutionOne mole of an ideal gas undergoes a process such that P∝1T. The molar heat capacity of this process is 4R.
View Solution
