Phenol is more acidic than :
C2H5OH
CH3COOH
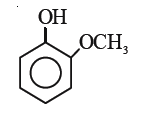
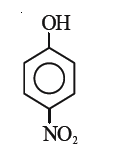
The correct Answer is:A
To determine which compound phenol is more acidic than, we need to analyze the acidic strength of phenol in comparison to the given options. The acidic strength of a compound is related to the stability of its conjugate base. The more stable the conjugate base, the stronger the acid.
1. Understanding Acidic Strength:
- Acidic strength can be assessed by looking at the stability of the conjugate base formed after the acid donates a proton (H⁺).
- For phenol (C₆H₅OH), when it donates a proton, it forms the phenoxide ion (C₆H₅O⁻).
2. Analyzing the Conjugate Base of Phenol:
- The conjugate base of phenol, the phenoxide ion, can stabilize the negative charge through resonance. This resonance involves the delocalization of the negative charge over the aromatic ring, increasing its stability.
3. Comparing with Ethanol (C₂H₅OH):
- The conjugate base of ethanol (C₂H₅O⁻) does not have resonance stabilization. The ethyl group (C₂H₅) is an electron-donating group, which increases electron density on the oxygen, making it less stable.
- Therefore, phenol is more acidic than ethanol.
4. Comparing with Acetic Acid (CH₃COOH):
- The conjugate base of acetic acid (CH₃COO⁻) can also stabilize the negative charge through resonance. However, the resonance in acetic acid is not as effective as in phenol due to the presence of the electron-donating methyl group.
- Thus, phenol is still more acidic than acetic acid.
5. Comparing with p-Nitrophenol:
- The conjugate base of p-nitrophenol (C₆H₄(NO₂)O⁻) is highly stabilized by the electron-withdrawing nitro group (NO₂), which enhances the stability of the conjugate base through resonance.
- Therefore, p-nitrophenol is more acidic than phenol.
Conclusion:
Based on the analysis, phenol is more acidic than ethanol (C₂H₅OH) and acetic acid (CH₃COOH), but less acidic than p-nitrophenol.
Final Answer: Phenol is more acidic than ethanol (C₂H₅OH).
- Acidic strength can be assessed by looking at the stability of the conjugate base formed after the acid donates a proton (H⁺).
- For phenol (C₆H₅OH), when it donates a proton, it forms the phenoxide ion (C₆H₅O⁻).
- The conjugate base of phenol, the phenoxide ion, can stabilize the negative charge through resonance. This resonance involves the delocalization of the negative charge over the aromatic ring, increasing its stability.
- The conjugate base of ethanol (C₂H₅O⁻) does not have resonance stabilization. The ethyl group (C₂H₅) is an electron-donating group, which increases electron density on the oxygen, making it less stable.
- Therefore, phenol is more acidic than ethanol.
- The conjugate base of acetic acid (CH₃COO⁻) can also stabilize the negative charge through resonance. However, the resonance in acetic acid is not as effective as in phenol due to the presence of the electron-donating methyl group.
- Thus, phenol is still more acidic than acetic acid.
- The conjugate base of p-nitrophenol (C₆H₄(NO₂)O⁻) is highly stabilized by the electron-withdrawing nitro group (NO₂), which enhances the stability of the conjugate base through resonance.
- Therefore, p-nitrophenol is more acidic than phenol.
Based on the analysis, phenol is more acidic than ethanol (C₂H₅OH) and acetic acid (CH₃COOH), but less acidic than p-nitrophenol.
Similar Questions
How would you account for the following:
(i) Phenol is more acidic than ethanol.
(ii) The boiling points of ethers are much lower than those of the alcohols of comparable molar masses.
(iii) Why do ethers possess dipole moment?
View SolutionAnswer the following :
(i) Phenol is more acidic than ethyl alcohol.
(ii) Phenol is an acid but does not react with NaHCO3.
(iii) Although phenoxide ion has more number of resonating structures than benzoate ino, benzoic acid is stronger acid than phenol . Why ?
(iv) Name the phenol with molecular formula C7H8O which on treatment with Br2 water readily gives a precipitate of C7h5OBr3.
(v) Unlike phenol, 2,4-dinitrophenol and 2,4,6-trinitrophenol are soluble in aqueous sodium carbonate solution.
(vi) Phenol has higher boiling than toluence. although they have almost the same molecular mass.
(vii)Ortho nitro phenol is steam volatile while para nitro phenol is not.
(viii) In Kolbe's reaction, instead of phenol, phenoxide ion is treated with CO2.
(ix) Nucleophilic substitution reactions are not very common in phenols.
(x) The dipole moment of phenol is smaller than that of methanol.
View Solution
Knowledge Check
Give the correct order of initials T or F for following statements Use T if statements is true and F if it is false.
Statement-I: p-chloro phenol is more acidic than p-flouro phenol.
Statement-II: T1/2 time period of (C6H5)3⊕C is less than T1/2 of C6H5⊕CH2
Statement-III: Solubility of Br− salt of alkali metals in water increases down the groupATFFBFFFCFTTDTFTPhenol are more acidic than alcohols because
Aphenoxide ion is stabilised by resonanceBphenols are more soluble in polar solventsCphenols ions do not exhibit resonanceDalcohols donot lose H atoms at all.Phenol is less acidic than :
Aacetic acidBp-methoxy phenolCacetyleneDethanol
Explain the following behaviours :
(i) Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses.
(ii) Ortho - nitrophenol is more acidic than ortho - methoxyphenol.
(iii) Cumene is a better starting material for the preparation of phenol.
View SolutionPhenol is less acidic than :
View SolutionPhenol is less acidic than
View SolutionPhenol is less acidic than
View SolutionPhenol is less acidic than
View Solution
