Identify the odd species out Which of the species among the following is different from others ?
The correct Answer is:B
To solve the problem of identifying the odd species out among the given options based on their aromatic or anti-aromatic nature, we will follow these steps:
Step 1: Understand the Criteria for Aromaticity and Anti-aromaticity
- Aromatic Compounds must satisfy the following conditions:
1. The molecule must be cyclic.
2. The molecule must be planar.
3. The molecule must follow Huckel's rule, which states that it must have 4n+2 π electrons, where n is a non-negative integer (0, 1, 2, ...).
- Anti-aromatic Compounds must satisfy:
1. The molecule must be cyclic.
2. The molecule must be planar.
3. The molecule must have 4n π electrons.
Step 2: Analyze Each Option
Let's analyze the given options one by one to determine whether they are aromatic or anti-aromatic.
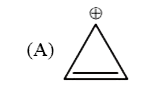
Option 1: Cyclobutadiene
- Structure: Cyclic with 4 π electrons (2 double bonds).
- Analysis:
- Cyclic: Yes
- Planar: Yes
- π Electrons: 4 (follows 4n rule, where n=1).
- Conclusion: Anti-aromatic
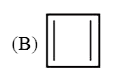
Option 2: Cyclobutene
- Structure: Cyclic with 4 π electrons (2 double bonds).
- Analysis:
- Cyclic: Yes
- Planar: Yes
- π Electrons: 4 (follows 4n rule, where n=1).
- Conclusion: Anti-aromatic
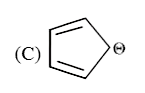
Option 3: Cyclopentadienyl Anion
- Structure: Cyclic with 6 π electrons (2 double bonds + 1 lone pair).
- Analysis:
- Cyclic: Yes
- Planar: Yes
- π Electrons: 6 (follows 4n+2 rule, where n=1).
- Conclusion: Aromatic
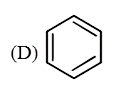
Option 4: Benzene
- Structure: Cyclic with 6 π electrons (3 double bonds).
- Analysis:
- Cyclic: Yes
- Planar: Yes
- π Electrons: 6 (follows 4n+2 rule, where n=1).
- Conclusion: Aromatic
Step 3: Identify the Odd Species
From our analysis:
- Option 1 (Cyclobutadiene) and Option 2 (Cyclobutene) are anti-aromatic.
- Option 3 (Cyclopentadienyl Anion) and Option 4 (Benzene) are aromatic.
Thus, the odd species out is Option 1: Cyclobutadiene, as it is the only one that is anti-aromatic.
Final Answer
The odd species out is Cyclobutadiene.
---
- Aromatic Compounds must satisfy the following conditions:
1. The molecule must be cyclic.
2. The molecule must be planar.
3. The molecule must follow Huckel's rule, which states that it must have 4n+2 π electrons, where n is a non-negative integer (0, 1, 2, ...).
1. The molecule must be cyclic.
2. The molecule must be planar.
3. The molecule must have 4n π electrons.
Let's analyze the given options one by one to determine whether they are aromatic or anti-aromatic.
- Structure: Cyclic with 4 π electrons (2 double bonds).
- Analysis:
- Cyclic: Yes
- Planar: Yes
- π Electrons: 4 (follows 4n rule, where n=1).
- Conclusion: Anti-aromatic
- Structure: Cyclic with 4 π electrons (2 double bonds).
- Analysis:
- Cyclic: Yes
- Planar: Yes
- π Electrons: 4 (follows 4n rule, where n=1).
- Conclusion: Anti-aromatic
- Structure: Cyclic with 6 π electrons (2 double bonds + 1 lone pair).
- Analysis:
- Cyclic: Yes
- Planar: Yes
- π Electrons: 6 (follows 4n+2 rule, where n=1).
- Conclusion: Aromatic
- Structure: Cyclic with 6 π electrons (3 double bonds).
- Analysis:
- Cyclic: Yes
- Planar: Yes
- π Electrons: 6 (follows 4n+2 rule, where n=1).
- Conclusion: Aromatic
From our analysis:
- Option 1 (Cyclobutadiene) and Option 2 (Cyclobutene) are anti-aromatic.
- Option 3 (Cyclopentadienyl Anion) and Option 4 (Benzene) are aromatic.
The odd species out is Cyclobutadiene.
Similar Questions
Knowledge Check
Identity the odd species out (which of the species among the following is different from others?)
A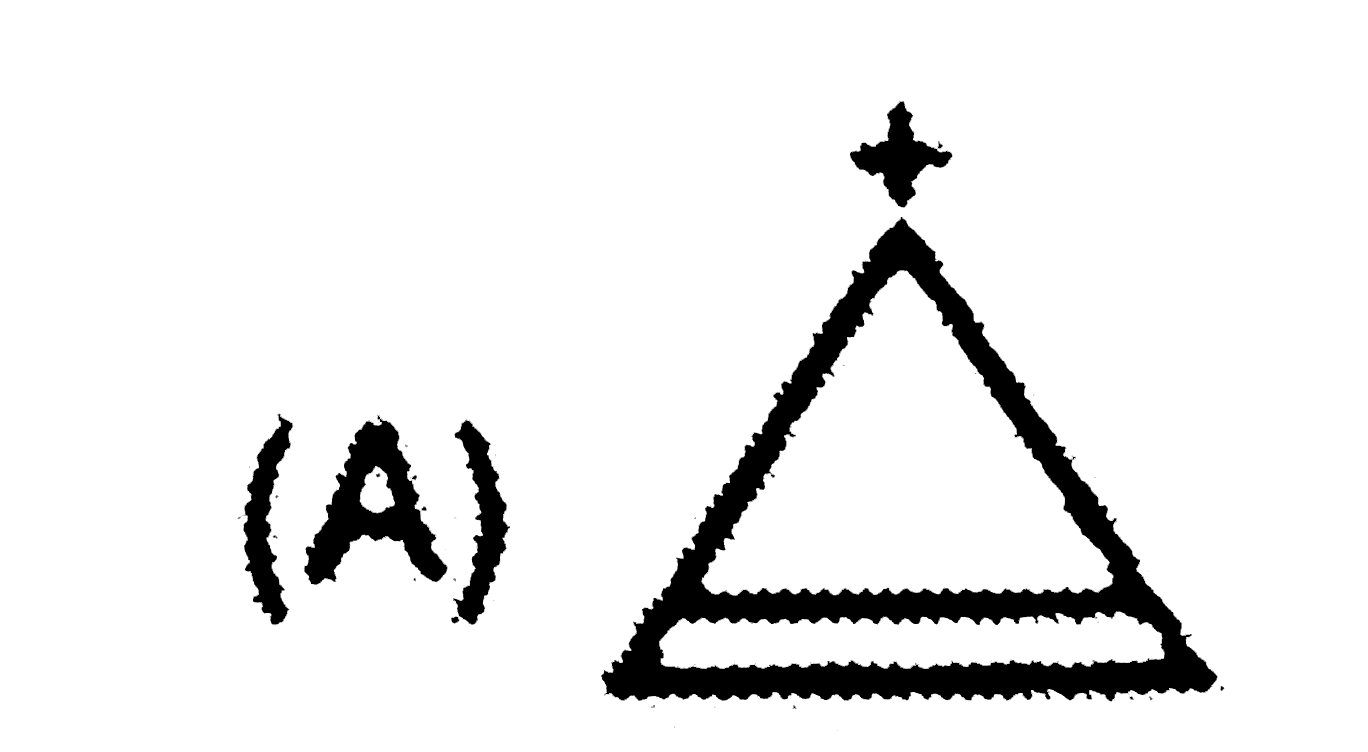 B
B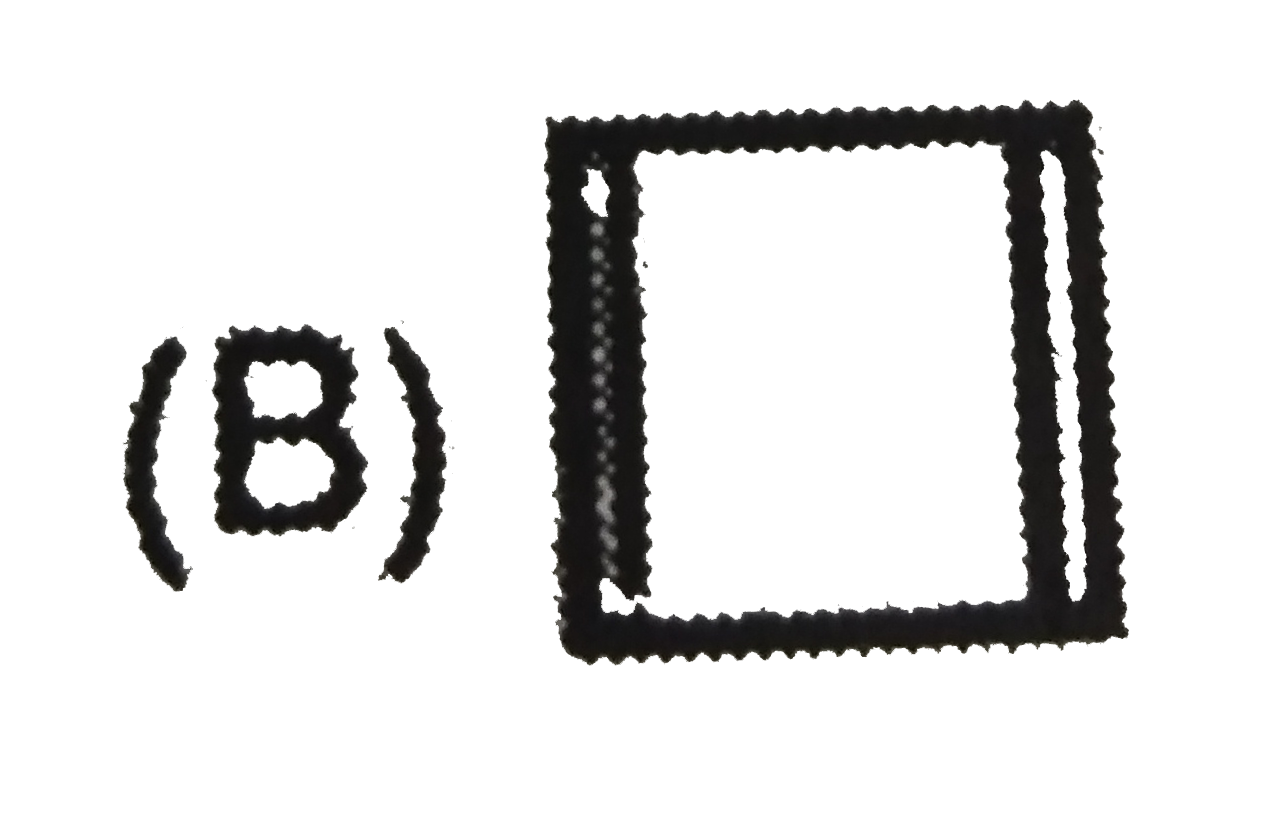 C
C D
D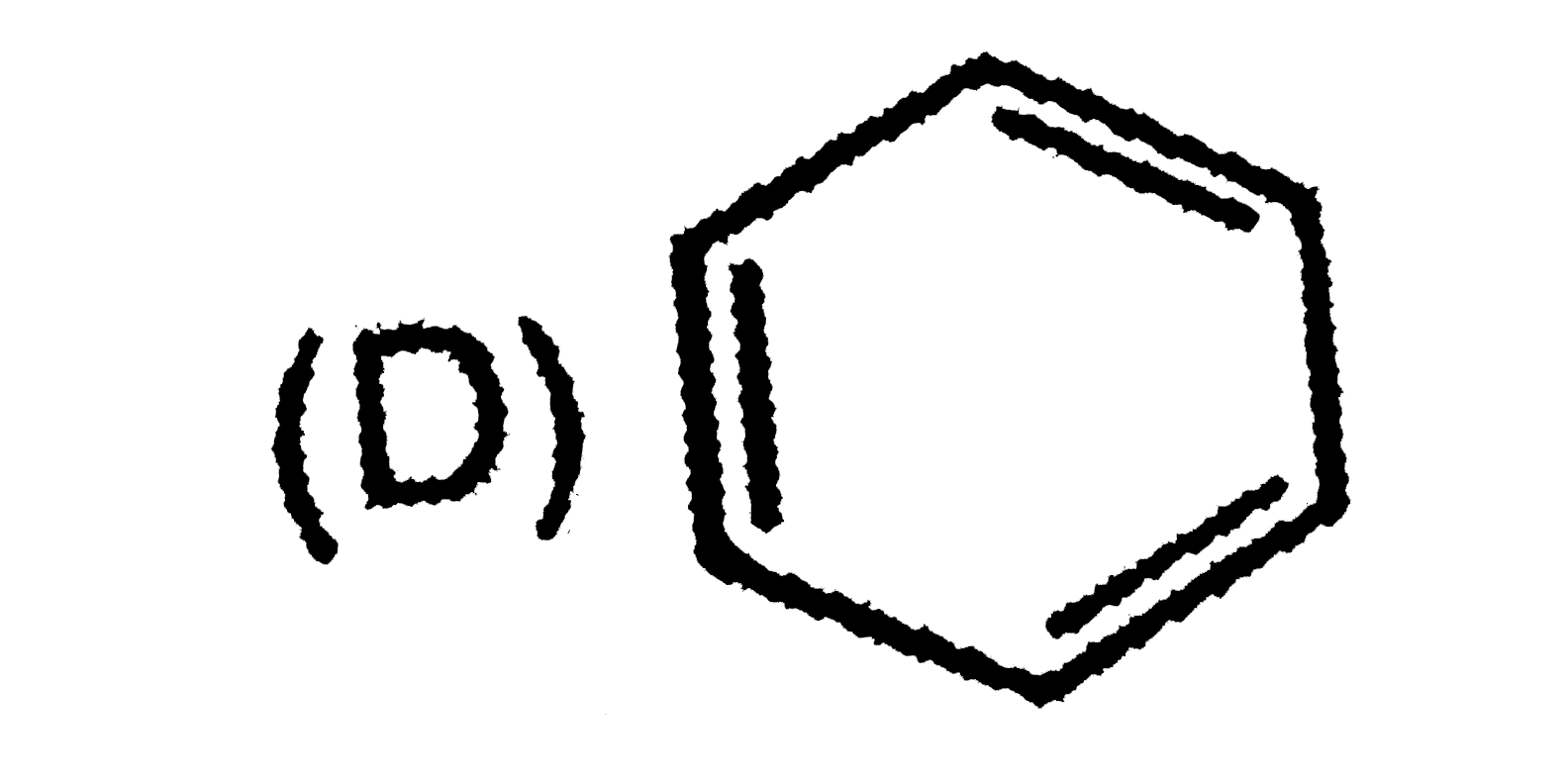
The achiral species among the following is :
Aan earBa screw driverCa screwDa handMost acidic species among the following is
A B
B C
C D
D
Most acidic species among the following is
View SolutionMost energetic species among the following is
View SolutionThe optically active species among the following is:
View SolutionThe optically active species among the following is:
View SolutionIdentify the chiral species among the following :1
View Solution

