Arrange the following compounds in order of increaing basicity.
(a). CH3NH2,CH3NH⊕3,CH3NH−
(b) CH3O−,CH3NH−,CH3CH−2.
The correct Answer is:2<1<3
Step by step video, text & image solution for Arrange the following compounds in order of increaing basicity. (a). CH_(3)NH_(2),CH_(3)NH_(3)^(o+),CH_(3)NH^(-) (b) CH_(3)O^(-),CH_(3)NH^(-),CH_(3)CH_(2)^(-). by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- GOCBook:MOTIONChapter:GOCExercise:Exercise - 4 Level - I
Explore 13 Videos
- GOCBook:MOTIONChapter:GOCExercise:Exercise - 4 Level - II
Explore 14 Videos
- GOCBook:MOTIONChapter:GOCExercise:Exercise - 2 (Level - II)
Explore 114 Videos
- GASEOUS STATE Book:MOTIONChapter:GASEOUS STATE Exercise:EXERCISE-4( LEVEL-II)
Explore 19 Videos
- GRIGNARD REAGENTBook:MOTIONChapter:GRIGNARD REAGENTExercise:EXERCISE-2
Explore 16 Videos
Similar Questions
Amongst the following the most basic compound is :
View SolutionFor 1-methoxy-1, 3-butadiene, which of the following resonating structure is least stable?
View SolutionWhich of the following statements is (are) true about resonance.
(a) Resonance is an intramolecular process.
(b) Resonance involves delocalization of both σ and π electrons.
(c )Resonance involves delocalization of π electrons and lone pair only.
(d) Resonance decreases potential energy of a molecule. (e ) Resonance has no effect on the potential energy of a molecule.
(f)Resonance is the only way to increase molecular stability.
(g) Resonance is not the only way to increase molecular stability.
(h) Any resonating molecule is always more stable than any nonresonating molecule.
(i) The canonical structure explains all features of a molecule.
(j) The resonance hybrid explains all features of a molecule.
(k)Resonating structures are real and resonance hybrid is imaginary.
(l) Resonance hybrid is real and resonating structures are imaginary.
(m) Resonance hybrid is always more stable than all canonical structures.
View SolutionThe average velocity of gas molecules is 400 m/sec calculate its rms velocity at the same temperature.
View SolutionWhich of the following compounds formed by the reaction sec-butylmagnesium iodide with a terminal alkyne
View SolutionWhich of the following is correct order of basic strength for the given compounds ?
(i)CH3−CH3−C−NH2∣∣NH (ii)CH3−C−CH3∣∣NH
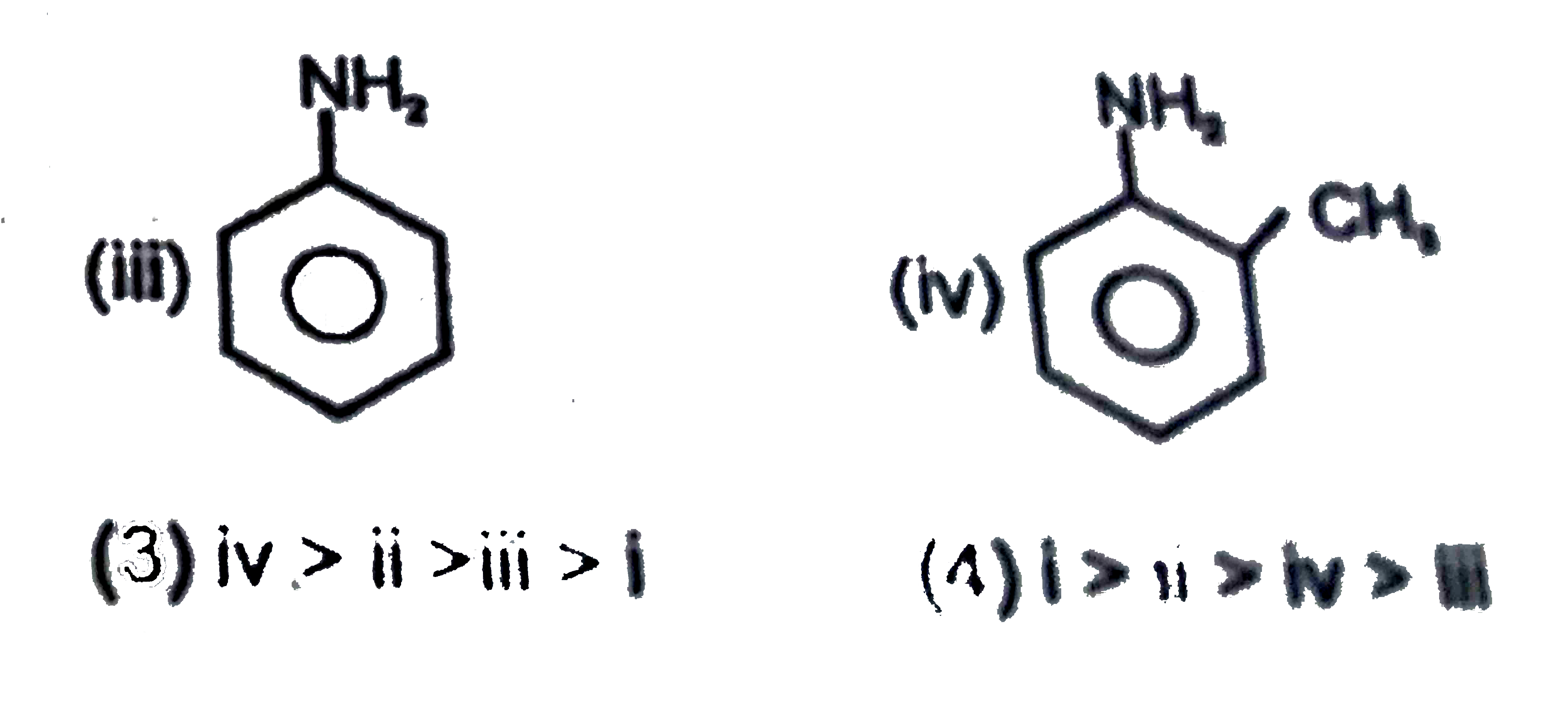
View SolutionHow many of the following compounds cleave diborane symmetrically? CH_(3)NH_(2), (CH_(3))_(2)NH, (CH_(3))_(3) N, CO(CH_(3))_(2)O, NH_(3)
View SolutionArrange the following in the increasing order of their basic strength CH_(3)NH_(2), (CH_(3))_(2)NH, (CH_(3))_(3)N, NH_(3)
View SolutionArrange the following compounds in order of increaing basicity. (a). CH_(3)NH_(2),CH_(3)NH_(3)^(o+),CH_(3)NH^(-) (b) CH_(3)O^(-),CH_(3)NH^(-),CH_(3)CH_(2)^(-) .
View SolutionThe correct order of basicities of the following compounds is - (I) , (II) CH_(3)CH_(2)NH_(2) : (III) (CH_(3))_(2)NH , (IV) CH_(3)-overset(O)overset(||)C-NH_(2))
View SolutionThe correct order of increasing basic nature for the bases NH_(3) CH_(3)NH_(2) and (CH_(3))_(2)NH is :
View SolutionThe increasing basicity order of the following compounds is : (A) " " CH_(3)CH_(2)NH_(2)" "(B) {:(" "CH_(2)CH_(3)),(" "|),(CH_(3)CH_(2)NH):} " "(C) H_(3)C-overset(CH_(3))overset(|)N-CH_(3)" "(D) Ph-overset(CH_(3))overset(|)N-H
View SolutionCH3NH2,(CH3)2NH,(CH3)3तथा NH3को इनकी क्षारकता के घटते क्रम मे लिखिए।
View SolutionArrange the following in the increasing order of their basic strengths :
CH3NH2,(CH3)2NH,(CH3)3N,NH3
View SolutionArrange the following in the increasing order of their basic strength in aqueous solutions: CH_(3)NH_(2), (CH_(3))_(3)N, (CH_(3))_(2)NH
View SolutionArnnge the following In the decreaalng order of theh basic strength in aqueous solution :
CH3NH2,(CH3)NH,(CH3)3N and NH3
View Solution
