30 " mL of " a solution containing 9.15gL−1
of an oxalate KxHy(C2O4)2.nH2O required for titration 27 " mL of " 0.12 N NaOH and 36 " mL of " 0.12 N KMnO4 for oxidation Find x,y,z. and n.
4
3
1
2
The correct Answer is:D
Step by step video, text & image solution for 30 " mL of " a solution containing 9.15gL^(-1) of an oxalate K_(x)H_(y)(C_2O_4)_2.nH_2O required for titration 27 " mL of " 0.12 N NaOH and 36 " mL of " 0.12 N KMnO_4 for oxidation Find x,y,z. and n. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- OXIDATION & REDUCTION Book:MOTIONChapter:OXIDATION & REDUCTION Exercise:EXERCISE -3(BALANCING OF REACTIONS )
Explore 56 Videos
- OXIDATION & REDUCTION Book:MOTIONChapter:OXIDATION & REDUCTION Exercise:EXERCISE -4(LEVEL-I)
Explore 14 Videos
- OXIDATION & REDUCTION Book:MOTIONChapter:OXIDATION & REDUCTION Exercise:EXERCISE-2 ( LEVEL-I)
Explore 50 Videos
- NOMENCLATURE OF ORGANIC COMPOUNDSBook:MOTIONChapter:NOMENCLATURE OF ORGANIC COMPOUNDSExercise:PREVIOUS YEAR
Explore 8 Videos
- P-BLOCK ELEMENTSBook:MOTIONChapter:P-BLOCK ELEMENTSExercise:Exercise - 4 | Level-I Previous Year | JEE Main
Explore 37 Videos
Similar Questions
Balance the following reactions by oxidation number method
KMnO4+H2SO4+HCl→K2SO4+MnSO4+H2O+Cl2
View SolutionHow many grams of H2O2 are required to convert 0.1 moles PbS to 0.1 moles PbSO4-
View SolutionWhich one is correctly matched :
View SolutionThe IUPAC name of the compound shown below
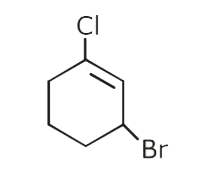
View SolutionThe number of hydrogen atom(s) attached to phosphorus atom in hypophosphorus acid is
View Solution30 " mL of " a solution containing 9.15gL−1
of an oxalate KxHy(C2O4)2.nH2O required for titration 27 " mL of " 0.12 N NaOH and 36 " mL of " 0.12 N KMnO4 for oxidation Find x,y,z. and n.
View SolutionA 100 mL solution of 0.1 N HCl was titrated with 0.2 N NaOH solution. The titration was discontinued after adding 30 mL of NaOH solution. The titration was completed by adding 0.25 N KOH solution. The volume of KOH required for completing the titration is :
View Solution10 mL of 1 N HCl, 25 mL of 2 N H2SO4 and 40 mL of X N HNO3 are mixed and made up to 2000 mL. 100 mL of this solution required 30 mL of NaOH taken from a solution containing 4 g of NaOH in 250 mL of solution. What is the value of X ?
View SolutionCalculate the pH value of a mixture containing 50 ml of 1 N HCl and 30 ml of 1 N NaOH solution, assuming both to be completely dissociated.
View SolutionA 100 ml solution of 0.1N-HCl was titrated with 0.2 N-NaOH solution. The titration was discontinued after adding 30 ml of NaOH solution. The remaining titration was completed by adding 0.25N-KOH solution. The volume of KOH required for completing the titration is
View SolutionFind the volume (in mL) of 0.2 (N) NaOH solution required to neutralise 25 mL of 0.2(N) H2SO4 solution.
View SolutionA 100ml solution of 0.1NHCl was titrated with 0.2? NNaOH solution. The titration. The remaining titration war completed by adding 0.25NKOH solution. The volume of KOH required for completing the titration is
View Solution1.575 g of oxalic acid (COOH)2.xH2O are dissolved in water and the volume made up to 250 mL. On titration 16.68 mL of this soltuion requires 25 mL of N15NaOH solution for complete neutralisation. Calculate x.
View Solution30 ml of a solution containing 9.15 gm/litre of an oxalate K_(x)H_(y) (C_(2)O_(4))_(z) nH_(2)O are rerquired for titrating 27 ml of 0.12 N NaOH and 36 ml of 0.12 N KMnO_(4) separately. Calculate x,y,z and n. Assume all H atoms (except H_(2)O ) are replaceable and x,y,z are in the simple ratio of gm atoms.
View SolutionWhat will be the normality of the salt solution obtained by neutralizing x mL of y (N) HCl with y mL of x (N) NaOH and finally adding (x + y) mL distilled water ?
View SolutionWhat will be the normality of the salt solution obtained by neutralizing x mL y (N) HCI with y mL x (N) NaOH, and finally adding (x + y) mL distilled water?
View Solution
