Statemen I : In Pb3O4 all Pb has +83oxidation number.
Statement II :Pb3O4 is mixed oxide of PbO & PbO2
If both Statement-I and Statement-II are true, and Statement-II is the correct explanation of Statement–I.
If both Statement-I and Statement-II are true but Statement-II is not the correct explanation of Statement–I.
If Statement-I is true but Statement-II is false.
If Statement-I is false but Statement-II is true.
The correct Answer is:D
Topper's Solved these Questions
- OXIDATION & REDUCTION Book:MOTIONChapter:OXIDATION & REDUCTION Exercise:EXERCISE -3(BALANCING OF REACTIONS )
Explore 56 Videos
- OXIDATION & REDUCTION Book:MOTIONChapter:OXIDATION & REDUCTION Exercise:EXERCISE -4(LEVEL-I)
Explore 14 Videos
- OXIDATION & REDUCTION Book:MOTIONChapter:OXIDATION & REDUCTION Exercise:EXERCISE-2 ( LEVEL-I)
Explore 50 Videos
- NOMENCLATURE OF ORGANIC COMPOUNDSBook:MOTIONChapter:NOMENCLATURE OF ORGANIC COMPOUNDSExercise:PREVIOUS YEAR
Explore 8 Videos
- P-BLOCK ELEMENTSBook:MOTIONChapter:P-BLOCK ELEMENTSExercise:Exercise - 4 | Level-I Previous Year | JEE Main
Explore 37 Videos
Similar Questions
Balance the following reactions by oxidation number method
KMnO4+H2SO4+HCl→K2SO4+MnSO4+H2O+Cl2
View SolutionHow many grams of H2O2 are required to convert 0.1 moles PbS to 0.1 moles PbSO4-
View SolutionWhich one is correctly matched :
View SolutionThe IUPAC name of the compound shown below
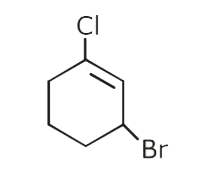
View SolutionThe number of hydrogen atom(s) attached to phosphorus atom in hypophosphorus acid is
View SolutionWhy following two reaction proced differently? Pb_(3)O_(4)+8HClrarr3PbCl_(2)+Cl_(2)+4H_(2)O and Pb_(3)O_(4)+4HNO_(3)rarr2Pb(NO_(3))_(2)+PbO_(2)+2H_(2)O
View SolutionWhat is the O.N of all the atoms in the following: (i)BrF_(3),(ii)Sb_(2)O_(5) ,(iii)Pb_(3)O_(4) ,(iv)(NH_(4))_(2)SO_(4)
View SolutionAssertion : Pb_(2)O_(4) react with HNO_(2) and form PbO_(2) Reason:Lead is stable in +4 oxidation state.
View SolutionPb_(3)O_(4)+HNO_(3)(dil.) overset(R.T.) to Pb(NO_(3))_(2)+PbO_(2)darr
View SolutionPb3O4 में Pb की ऑक्सीकरण संख्या कीजिए।
View SolutionWhich among the following oxides of lead is strong oxidizing agent? PbO,PbO2 ,Pb3O4
View SolutionWhy do the following reactions proceed differently? Pb3O4+8HCl→3PbCl2+Cl2+4H2O and Pb3O3+4HNO3→2Pb(NO3)2+PbO2+2H2O
View SolutionCalculate the oxidation number of Pb In Pb3O4
View SolutionPb3O4+4HNO3→2Pb(NO3)2+PbO2+2H2O Which is true about the lead ions in Pb3O4 ?
View SolutionPb3O4 is regarded as a compound oxide of PbO and PbO2. How many parts of PbO2, are present in it?
View Solution

