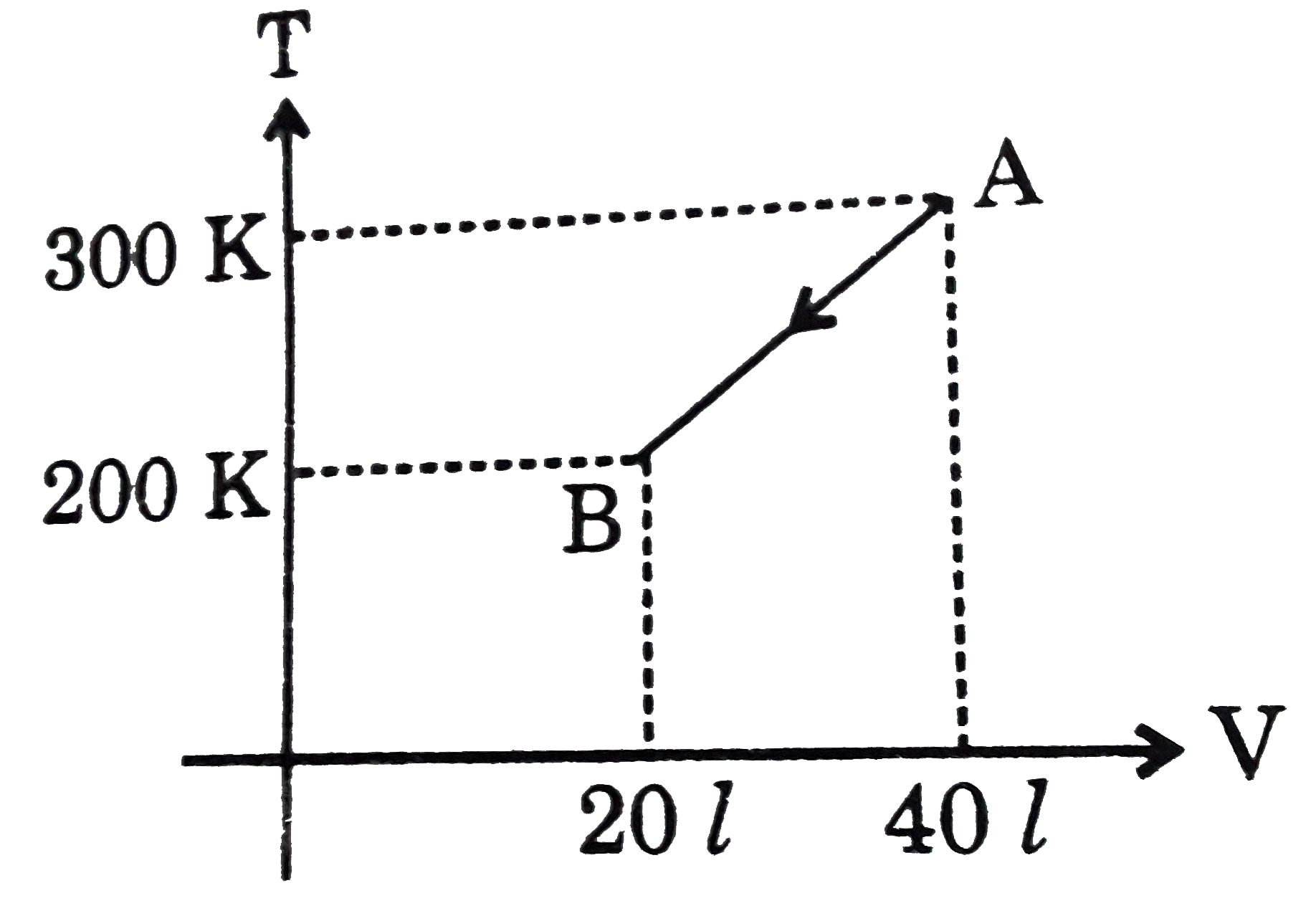
Figure shows the variation of the internal energy U with the density ρ of an ideal monoatomic gas for a therodynamic process AB. Process AB is a part of arectangular hyperbola. Find the work done (in joule) by gas in process AB.
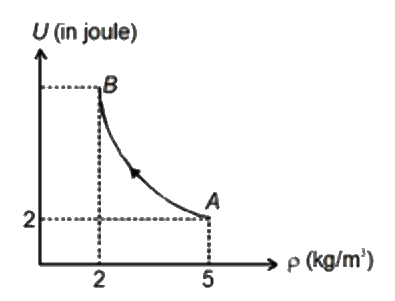
The correct Answer is:2
Step by step video & image solution for Figure shows the variation of the internal energy U with the density rho of an ideal monoatomic gas for a therodynamic process AB. Process AB is a part of arectangular hyperbola. Find the work done (in joule) by gas in process AB. by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
The graph shows variation of internal energy U with density ρ of one mole of an ideal monatomic gas. The process AB is a part of rectangular hyperbola. Find the work done (in J ) in the process.
'(##CEN_KSR_PHY_JEE_C15_E01_024_Q14##)'
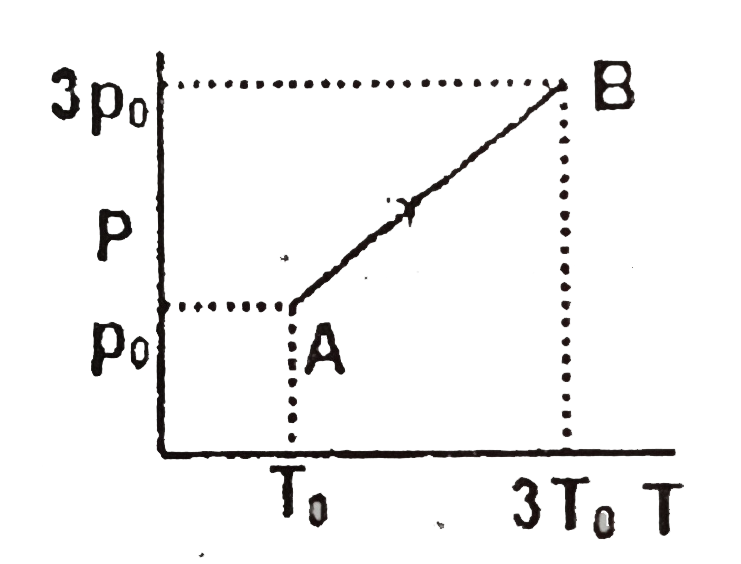
View SolutionTwo moles of a monoatomic ideal gas undergoes a process AB as shown in the figure. Then, find the work done during the process in Joule.
View Solution
Knowledge Check
Figure shows the variations of the internal energy U With density ρ one mole an ideal monatomic gas for thermodynamic cycle ABCA . Here process AB is a part of rectangular hyperbola,-
_S01_092_Q01.png) Aprocess AB is isothermal & net work in cycle is done by gas.Bprocess AB is isobaric & net work in cycle is done by gas.Cprocess AB is isobaric & net work in cycle is done on the gas.Dprocess AB is adiabatic & net work in cycle is done by gas.
Aprocess AB is isothermal & net work in cycle is done by gas.Bprocess AB is isobaric & net work in cycle is done by gas.Cprocess AB is isobaric & net work in cycle is done on the gas.Dprocess AB is adiabatic & net work in cycle is done by gas.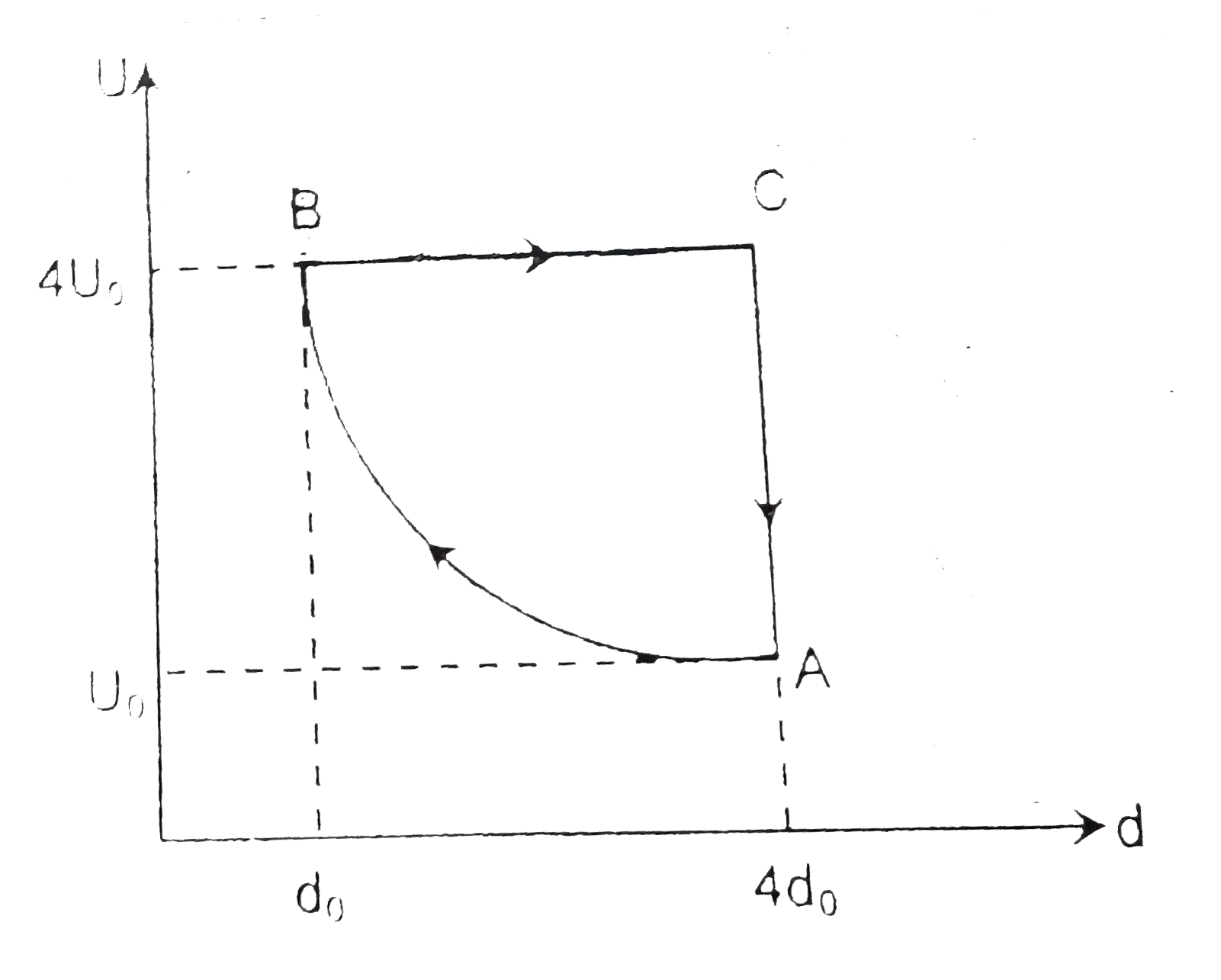
The variation of internal energy U and density d of one mole of an ideal monatomic gas is shown inn cyclic process ABCA. The curve from A to B is a rectangular hyperbola. Select correct alternatives.AThe work done by gas in process AB is 2U0BThe work done by gas in process BC is −3U02ln2CThe molar heat capacity of gas for process CA is 3R2DThe molar heat capacity of gas for process AB is 3R2Find work done by the gas in the process shown in figure.
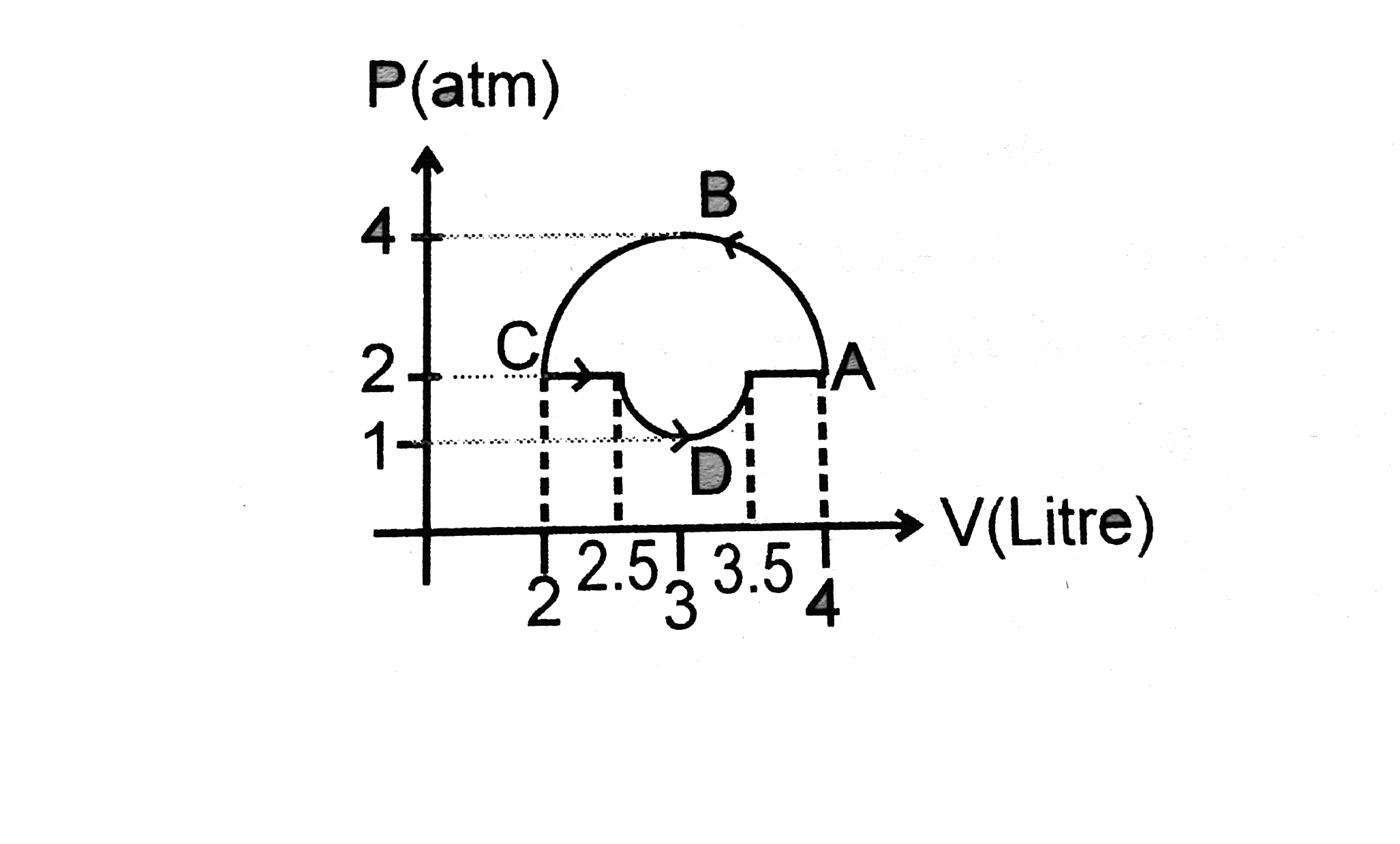 A52πatmLB52atmLC−32πatmLD−54πatmL
A52πatmLB52atmLC−32πatmLD−54πatmL
Find work done by the gas in the process shown in figure :
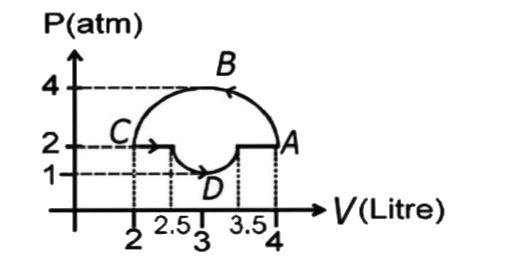
View SolutionDuring the process AB of an ideal gas
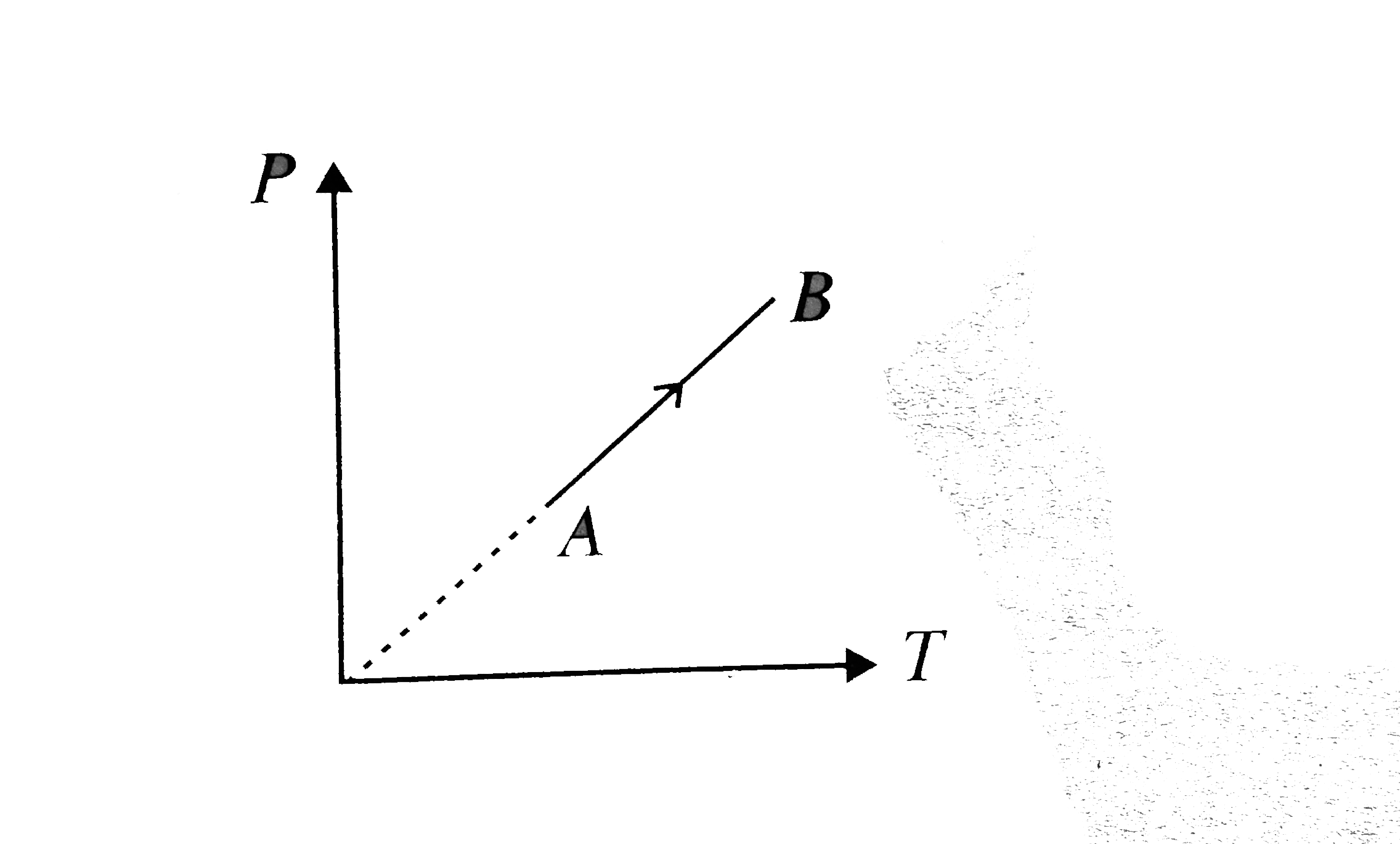
View Solution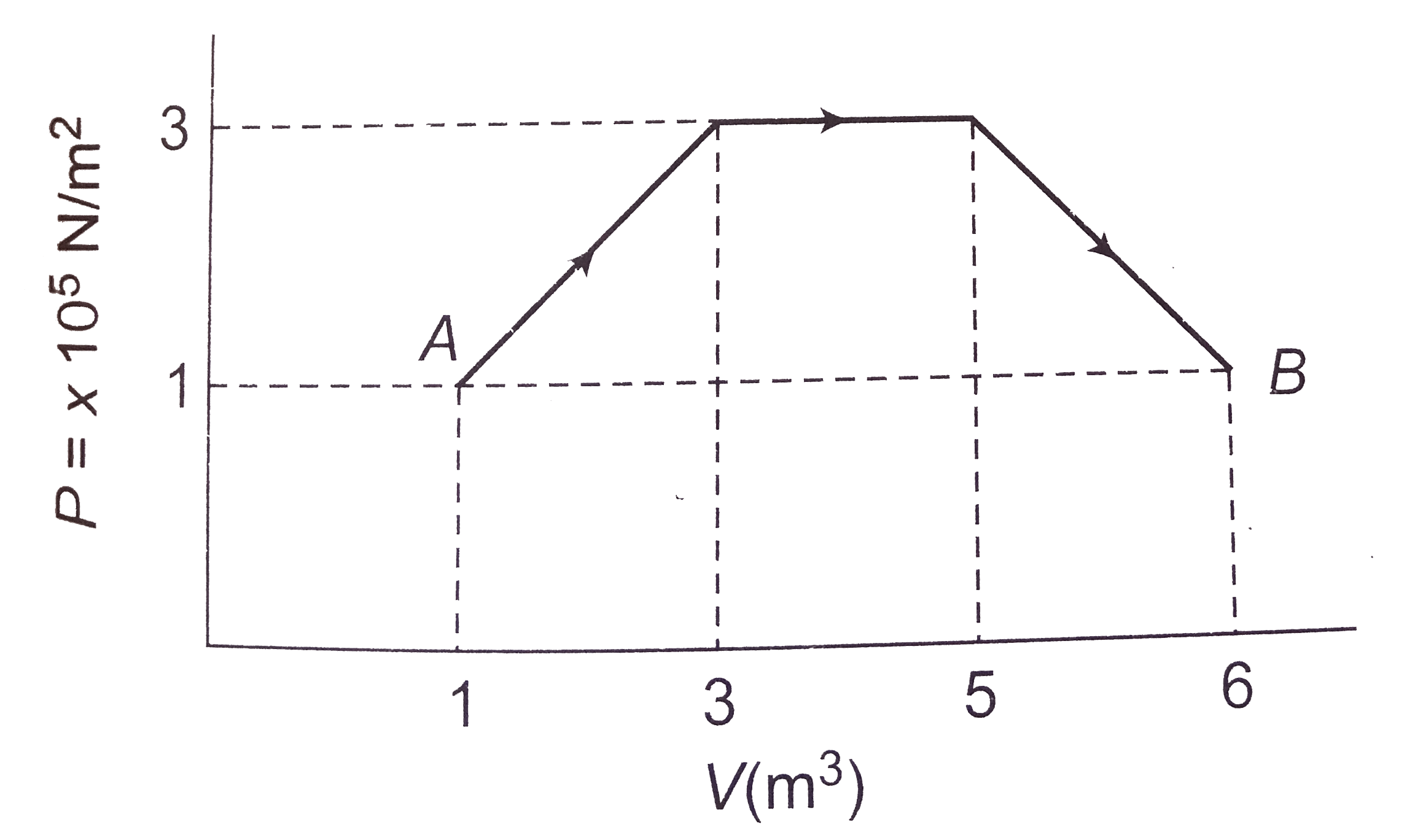
The work done in the process AB is
View SolutionAn ideal monoatomic gas undergoes a cyclic process ABCA as shown in the figure. The ratio of heat absorbed during AB to the work done on the gas during BC id
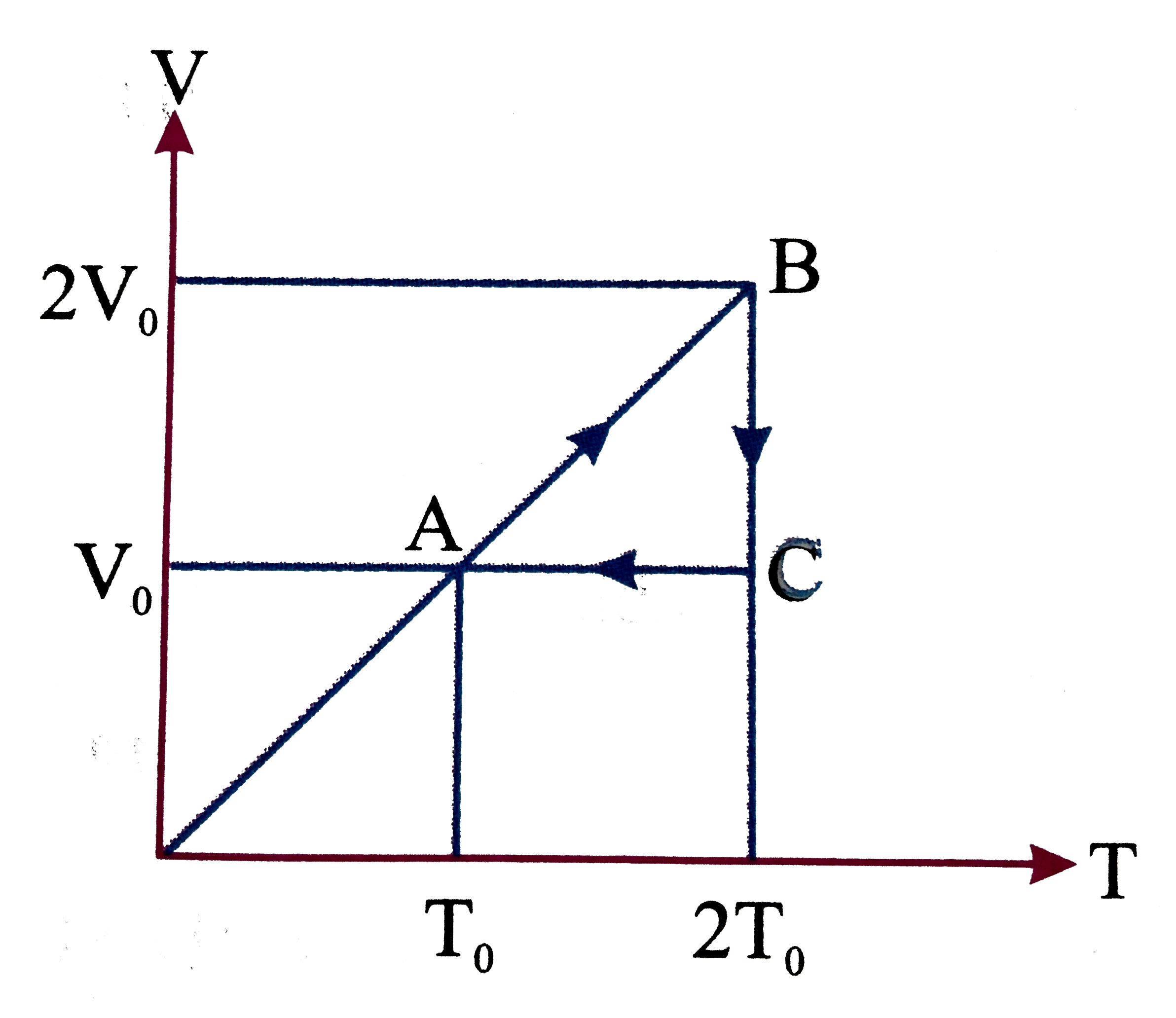
View SolutionFor 1 mol of an ideal gas work in process AB will be:
View Solution
