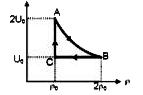
The graph shows variation of internal energy U with density ρ of one mole of an ideal monatomic gas. The process AB is a part of rectangular hyperbola. Find the work done (in J ) in the process.
'(##CEN_KSR_PHY_JEE_C15_E01_024_Q14##)'
The correct Answer is:2
Topper's Solved these Questions
Similar Questions
Thegraph AB shown in the figure is a plot of temperature of a body in degree Celsius and degree Fahrenheit. If the slope of line'AB is xy, find (x+y).
'(##CEN_KSR_PHY_JEE_C13_E01_001_Q01##)'
View SolutionIn a single slit diftion, slit width is 0.6mm and distance of first minima from central position is 1mm. If screen distance is 1.2m, then wavelength of light (in nm) is
View SolutionAn ideal monoatomic gas undergoes a process in which its internal energy U and density ρ vary as Uρ= constant. The ratio of change in internal energy and the work done by the gas is
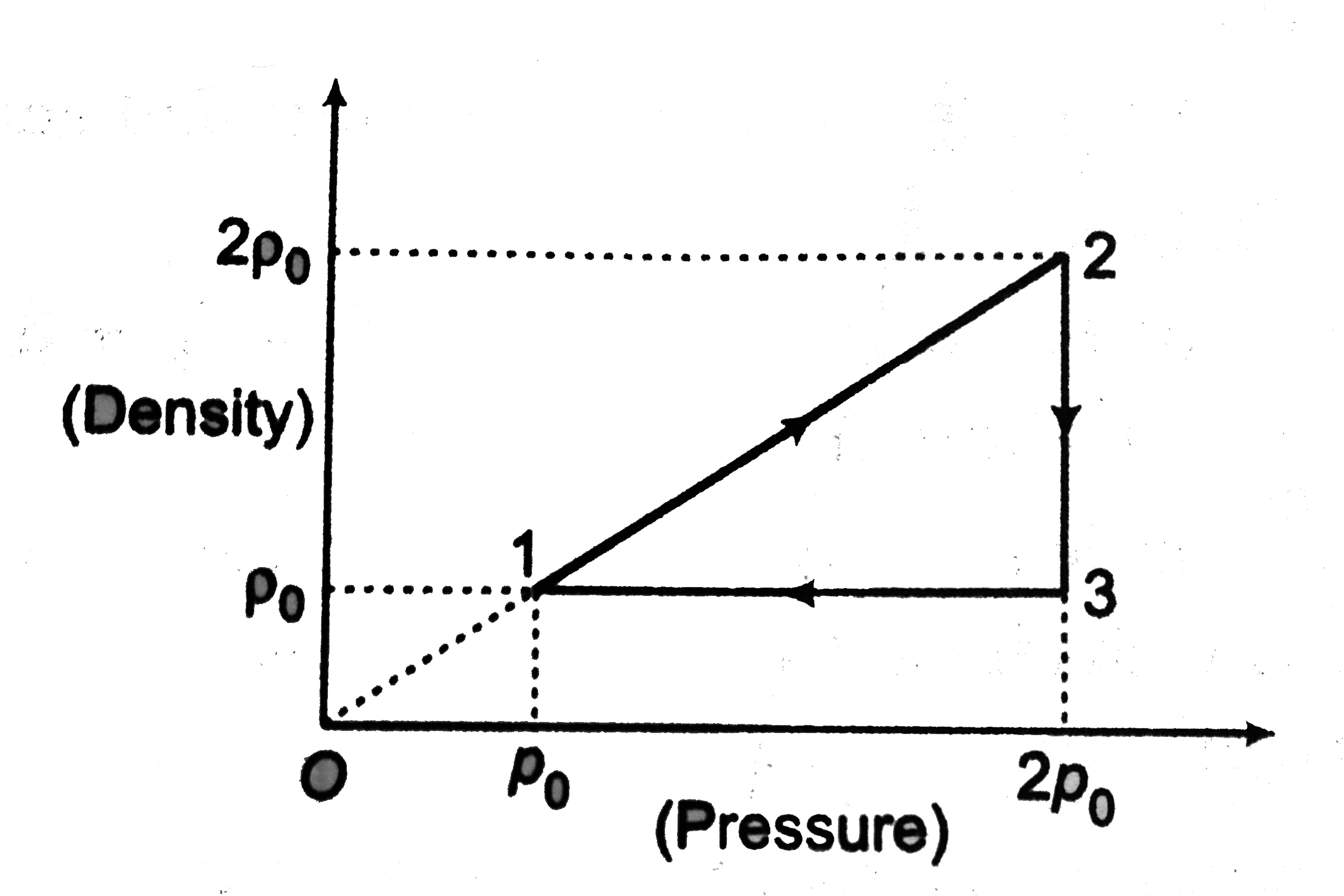
View SolutionThe density (rho) versus pressure (p) graph of one mole of an ideal monoatomic gas undergoing a cyclic process is shown in figure. Molecular mass of gas is M. (a) Find work done in each process. (b) Find heat rejected by gas in one complete cycle. (c) Find the efficiency of the cycle.
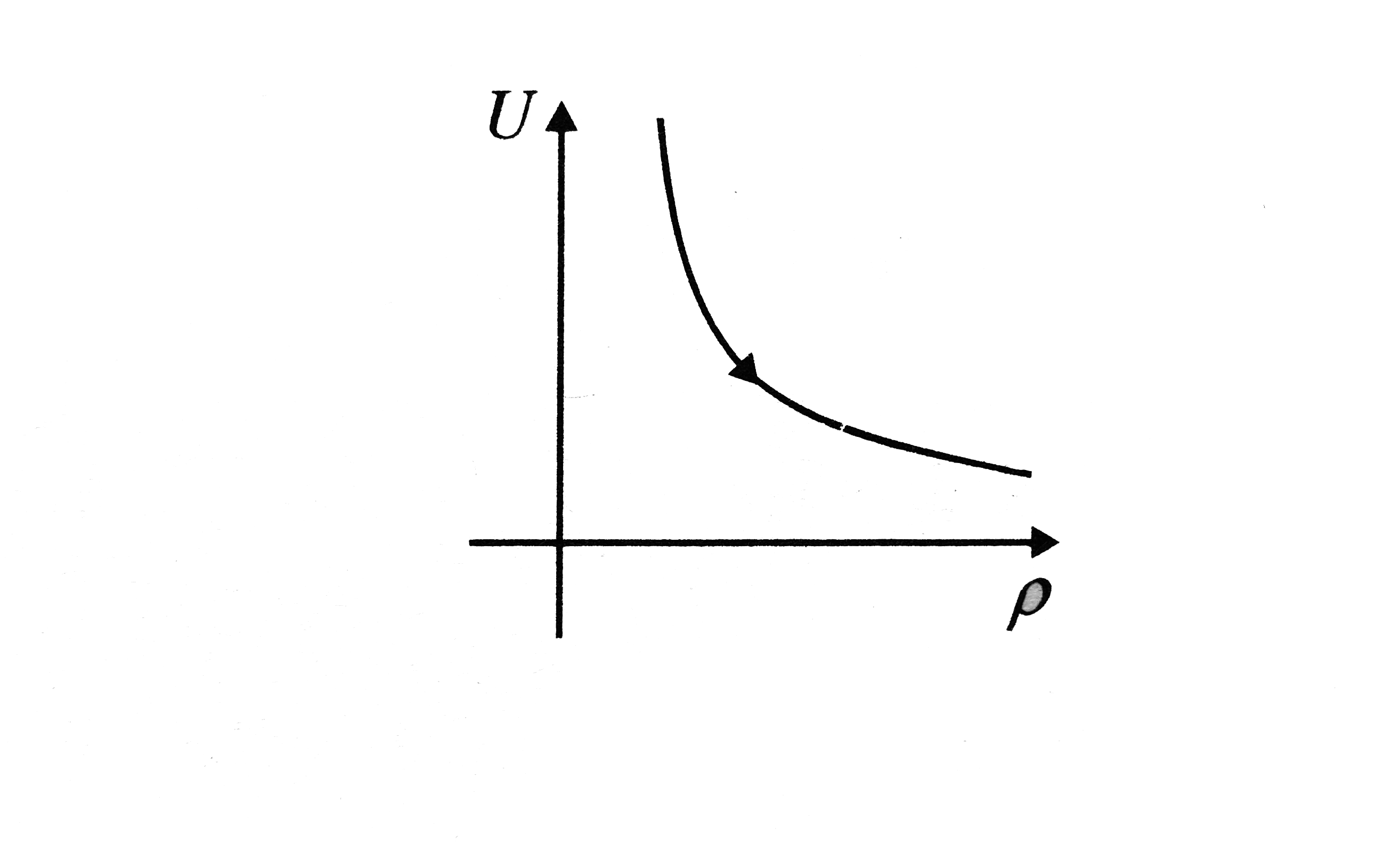
View SolutionVariation of internal energy with density of 1mole of monatomic gas is depicted in Fig. Corresponding variation of pressure with voluem can be depicted as (assume the curve is rectangular hyperbola)
View SolutionThe variation of internal energy U and density d of one mole of an ideal monatomic gas is shown inn cyclic process ABCA. The curve from A to B is a rectangular hyperbola. Select correct alternatives.
View SolutionFigure shows the variations of the internal energy U With density rho one mole an ideal monatomic gas for thermodynamic cycle ABCA . Here process AB is a part of rectangular hyperbola,-
View SolutionFigure shows the variation of the internal energy U with the density rho of an ideal monoatomic gas for a therodynamic process AB. Process AB is a part of arectangular hyperbola. Find the work done (in joule) by gas in process AB.
View SolutionAn ideal monoatomic gas undergoes a process in which its internal energy U and density rho vary as Urho = constant. The ratio of change in internal energy and the work done by the gas is
View SolutionThe density (ρ) versus pressure (p) graph of one mole of an ideal monoatomic gas undergoing a cyclic process is shown in figure. Molecular mass of gas is M.
(a) Find work done in each process.
(b) Find heat rejected by gas in one complete cycle.
(c) Find the efficiency of the cycle.
View SolutionAn ideal monoatomic gas undergoes a process in which its internal energy U and density rho vary as Urho = constant. The ratio of change in internal energy and the work done by the gas is
View SolutionVariation of internal energy with density of 1 "mole" of monatomic gas is depicted in Fig. Corresponding variation of pressure with voluem can be depicted as (assume the curve is rectangular hyperbola)
View Solutionएक आदर्श गैस के लिए चक्रीय प्रक्रम ABCA है। AB, BC व CA प्रक्रम में आदर्श गैस को 50 J, 20 J व 5Jऊष्मा दी जाती है। प्रक्रम AB में गैस की आन्तरिक ऊर्जा 60J से बढ़ती है एवं प्रक्रम BC में गैस द्वारा किया गया कार्य 30J है। प्रक्रम CAमें गैस की आन्तरिक ऊर्जा में वृद्धि है :
View Solutionचित्र में एक पर्मानुक आदर्श गैस के चक्रीय प्रक्रम के लिए आन्तरिक ऊर्जा (U) तथा घनत्व (ρ) में परिवर्तन दर्शाया गया है। AB आयताकार अति परवलय का एक भाग है, अर्थात U×ρ= अचर है। तब
View SolutionAn ideal monoactomic gas undergoes a process ini which its internal energy depends upon its volume as U=asqrt(V) [where a is a constant] (a) find work done by a gas and heat transferred to gas to increse its internal energy by 200 J. (b) find molar specific heat of gas for this process.
View Solution

