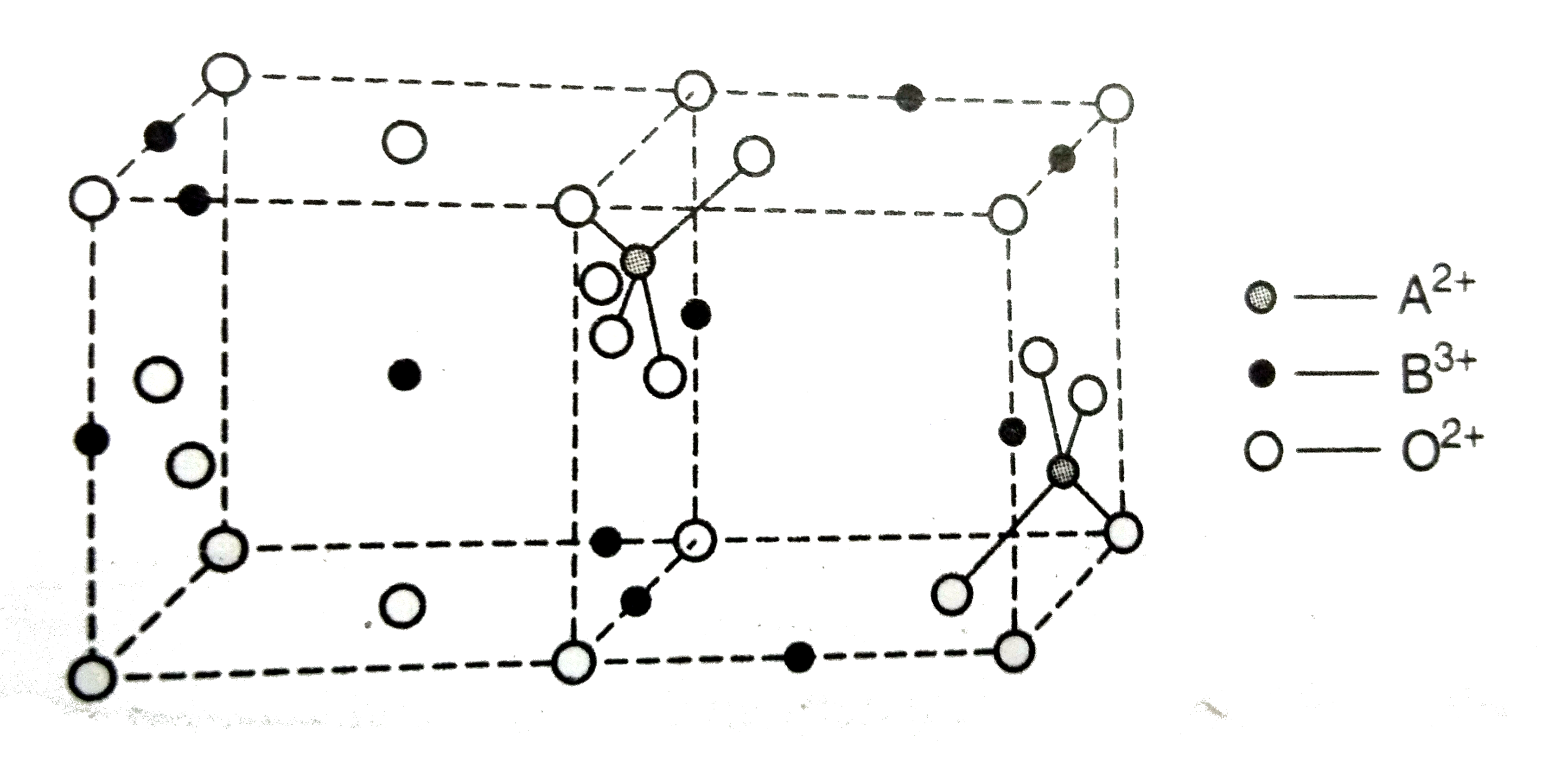
Fraction of the total octahedral voids occupied will be :
12
14
18
16
The correct Answer is:A
Topper's Solved these Questions
Similar Questions
In chromium (III) chloride CeCl3 chloride ions have cubic close packed arrangement and Cr (III) ions present in the octahedral voids. What fraction of the octahedral void is occupied ? What fraction of the total number of voids is occupied?
View SolutionIn chromium (III) chloride CeCl3 chloride ions have cubic close packed arrangement and Cr (III) ions present in the octahedral voids. What fraction of the octahedral void is occupied ? What fraction of the total number of voids is occupied?
View Solution
Knowledge Check
In a compound XY2O4 , oxide ions are arranged in CCP and cations X are present in octahedral voids. Cations Y are equally distributed between octahedral and tetrahedral voids. The fraction of the octahedral voids occupied is :-
A1/2B1/4C1/8D1/6The empirical formula of a mixed oxide. In which the oxide ions are present in the CCP lattice positions, half of the octahedral voids are occupied by trivalent ions Y3+ and one-fifth of tetrahedral voids are occupied by divalent X2+ ions, will be
AXY2O4BX2YO4CX4Y5O10DX5Y4O10
In chromium (III) chloride CeCl3 chloride ions have cubic close packed arrangement and Cr (III) ions present in the octahedral voids. What fraction of the octahedral void is occupied ? What fraction of the total number of voids is occupied?
View SolutionWhat is the C.N. of octahedral void ?
View SolutionIn a sodium chloride structure, the percentage of the octahedral voids occupied by cation is:
View SolutionAn octahedral void is surrounded by
View SolutionIn a crystalline solid, having formula AB2O4 oxide ions are arranged in cubic close packed lattice while cations A are present in tetrahedral voids and cations B are present in octahedral voids.
(a) What percentage of the tetrahedral voids is occupied by A?
(b) What percentage of the octahedral voids is occupied by B ?
View SolutionIn solid, oxide ions are arranged in ccp. One sixth of the tetrahedral voids are occupied by the cations (A) while one third of the octahedral voids, are occupied by the cations (B). What is the formula of the compound ?
View Solution

