In SF4 the type of hybridization shown by S is
A
sp3d2
B
sp3d
C
sp3d3
D
sp3
Video Solution
Text Solution
The correct Answer is:B
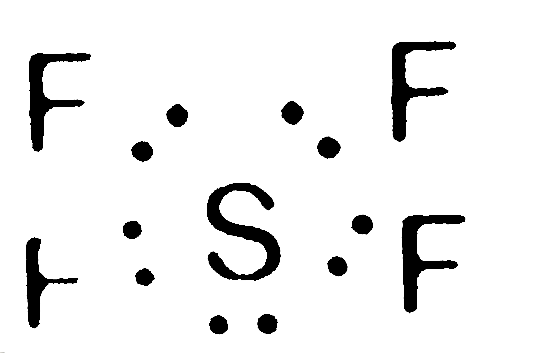
There are five pairs of electrons around S in SF4 . Therefore state of hybridisation of S is sp3d (Use VSEPR theory ).
|
Similar Questions
Knowledge Check
The type of hybridization in water molecule is
AspBsp2Csp3DNone.The type of hybrid orbitals used by OF2 is
Adsp2Bsp3Csp2DspIn allene (C3H4), the type (s) of the carbon atom (s) is (are) :
Asp and sp3Bsp and sp2Conly sp2Dsp2 and sp3
In allene (C3H4), the type(s) of hybridisation of the carbon atoms, is (are)
View SolutionThe type of hybridization of boron in diborane is
View Solution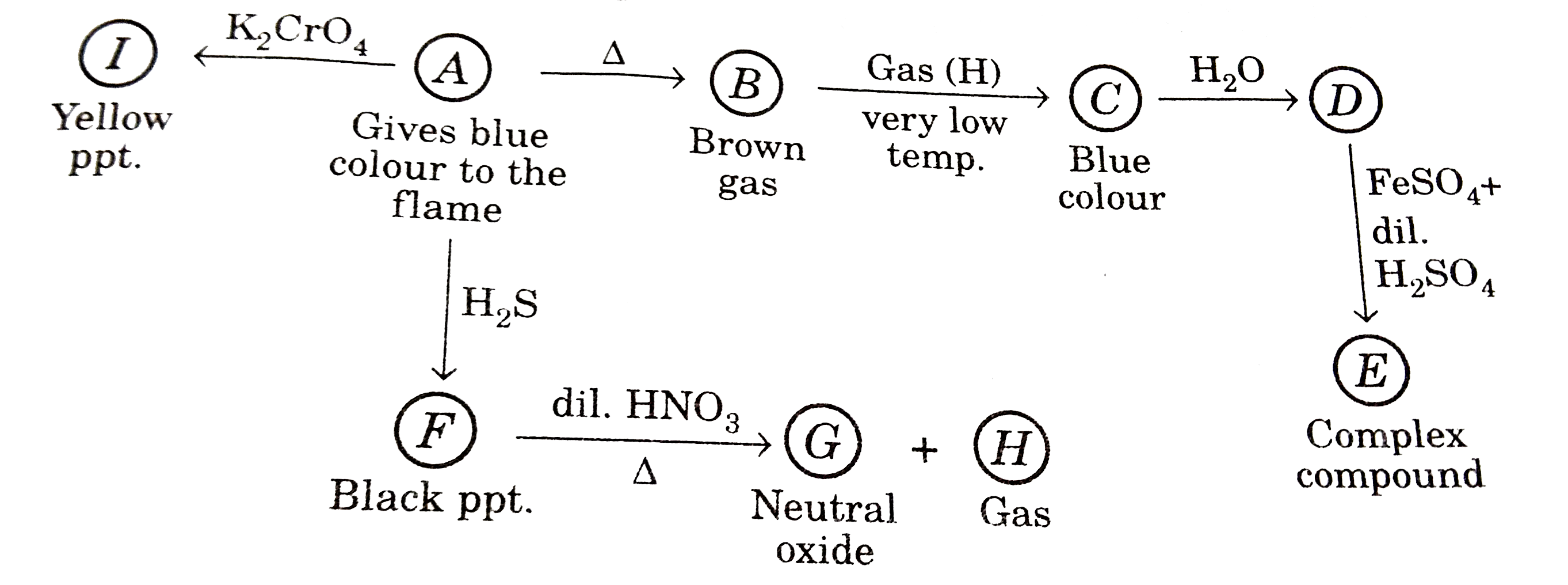
Type pf hybridization of complex (E):
View SolutionIn which of the following molecules , the type of hybridization changes when
View SolutionWhat is the hybridization of S in SF6 ?
View Solution