The stability of interhalogen compounds follows the order
A
IF3>BrF3>CIF3
B
BrF3>IF3>CIF3
C
CIF3>BrF3>IF3
D
CIF3>IF3>BrF3
Video Solution
Text Solution
The correct Answer is:A
The stability of interhalogens increases as the size of difference and polarity of bond increases. Thus the correct order of stability is
IF3>BrF3>CIF3
|
Similar Questions
Knowledge Check
Interhalogen compounds are
Acovalent moleculesBdiamagnetic in natureCvolatile solids/liquids at 298 K except CIFDAll of the aboveInterhalogen compounds are
Acovalent compoundsBionic compoundsCmore reactive than halogen moleculesDless reactive than halogen molecules.T-shaped interhalogen compounds is
ACIF3BICICCIF5DIF5
Explain the structure of interhalogen compound of the type XX5.
View SolutionThe numerous interhalogen compounds are
View SolutionWhich of the following form of interhalogen compounds does not exist ?
View SolutionThe stability of the hydrides follows the order
View SolutionThe Stability of
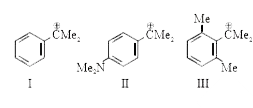
Follow the order
View Solution

