Which of the following complex ions has geometrical isomers?
A
∣∣Co(en)3∣∣3+
B
∣∣Ni(NH3)5Br∣∣+
C
∣∣Co(NH3)2en2∣∣3+
D
∣∣Cr(NH3)4(en)∣∣3+
Video Solution
Text Solution
The correct Answer is:C
Geometrical isomerism is shown by disubstituted octahedral complexes such as [Co(NH3)2(en)2]3+
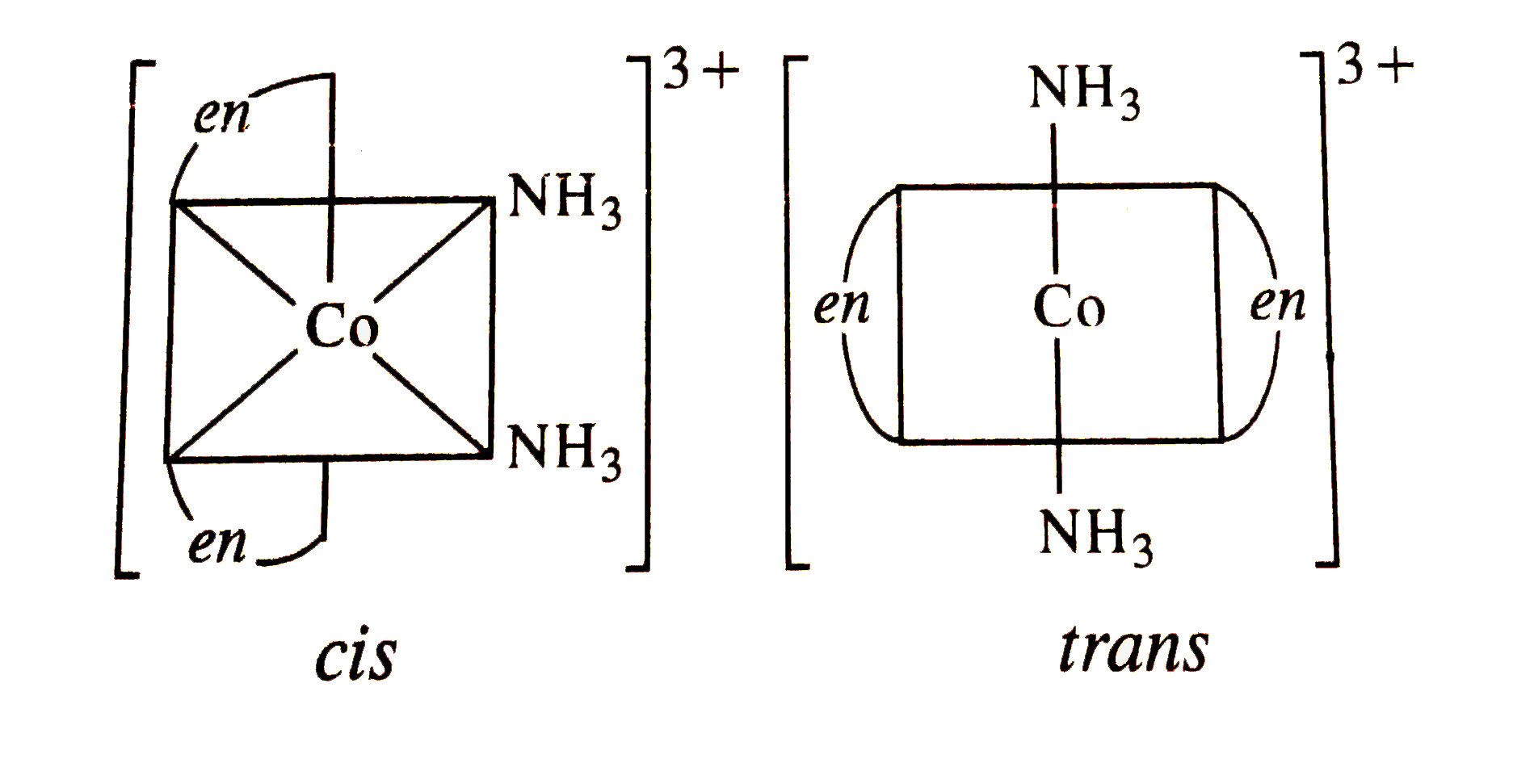
|
Similar Questions
Knowledge Check
Which of the following complex ions has geometrical isomers?
A[Co(en)3]3+BNi(NH3)5Br]+C[Co(NH3)2(en)2]3+D[Cr(NH3)4(en)]3+Which of the following complexes have geometrical isomers that are optically inactive due to the pressence of centre of symmetry ?
A[Pt(bn)2]2+B[Fe(gly)3]0C[Cr(H2O)3Cl3]0D[Pd{NH2−CH(CH3')−CO2}2]2.Which of the following compounds exhibits geometrical isomers-
AC2H5BrB(CH)2(COOH)2CCH3CHOD(CH2)2(COOH)2
Which of the following complex does not show geometrical isomerism ?
View SolutionWhich of the following does not exist as geometric isomers ?
View SolutionWhich of the following complex will show geometrical as well as optical isomers?
View SolutionWhich of the following complexes are polymerisation isomers ?
View SolutionWhich of the following complexes are polymerisation isomers ?
View Solution
