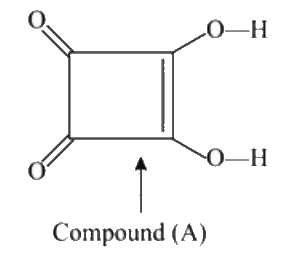
Consider the following compound A (below) Select the correct statement (s)
A
It is more acidic that CH3OH
B
it is more acidic than CH3O∣∣COH
C
it reacts very fast with Lucas reagent
D
It is a diacidic base
Text Solution
The correct Answer is:A, B
−Compound which donate H⊕ are acid.
− Acidity of acids increases as stability of conjugate base increases.
− Due to resonance stabilization of conjugate base of compound, it is more acidic than CH3−OH & CH3−COOH.
|
Similar Questions
Knowledge Check
Select the the correct statement (s)
AMilk is a type of emulsionBBrownian motion is observed in emulsionCCleansing action of soap is due to formation of emulsions (Micelle)DAll of the above are correct statements
Select correct statement (s) :AA is OsO4/aq.NaHCO3.BB is mCPBA/H3O⊕.CC is obtained when D is heated with conc. H2SO4.DReaction C to Z is stereo non-selective.Select correct statement (s) :
Astrongest force of attraction in alkyl halide is the London forceBLondon fore is a surface attractionCMolecules with larger surface area have higher b.pDAll of the above are correct statements