Consider the reactions shown below:
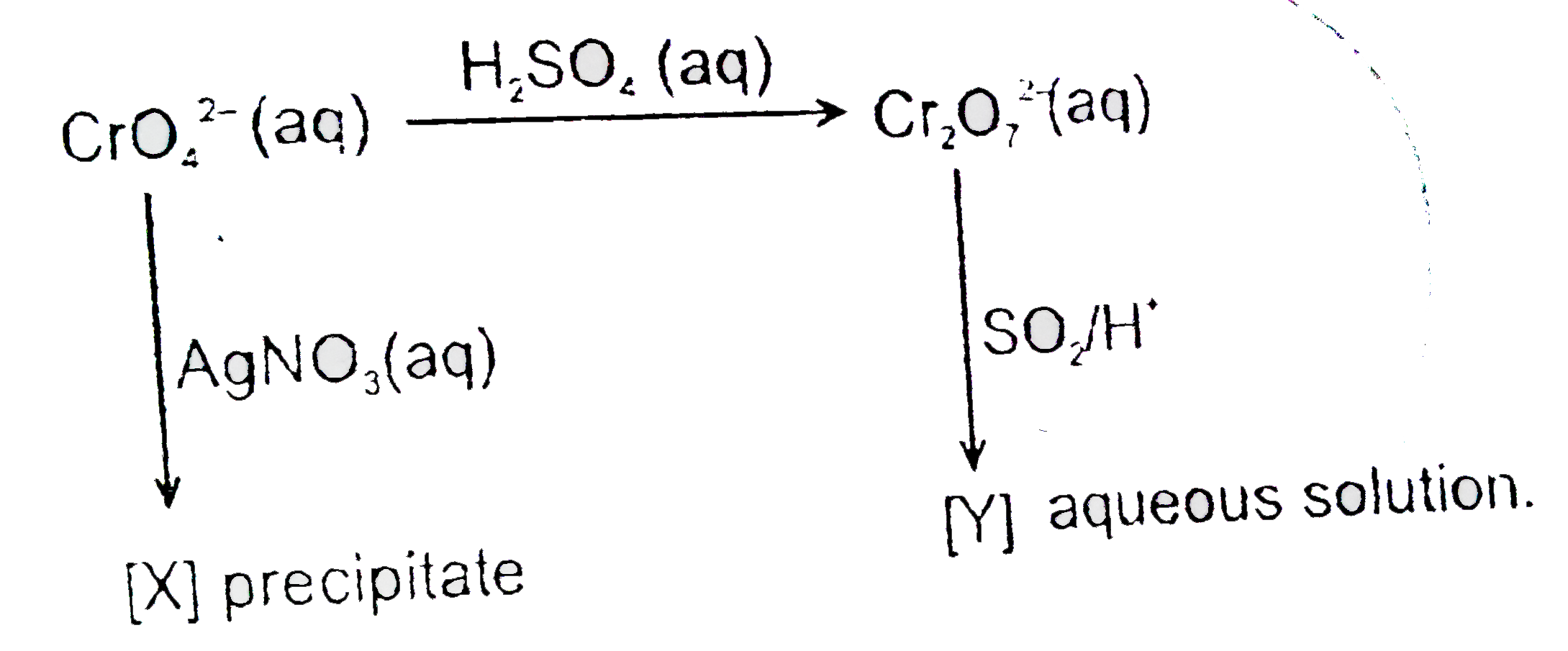
Which of the following statement (s) is/are correct?
A
[X] is a yellow coloured precipitate.
B
[X] is soluble in ammonia solution
C
[Y] gives green coloured solution with excess of sodium hydroxide solution.
D
The conversion of Cr2O2−7 to [Y] is an redox reaction.
Video Solution
Text Solution
The correct Answer is:B,C,D
(A)Ag+2CrO2−4→Ag2CrO4⏐⏐↓(red)[X]
(B)Ag2CrO4⏐⏐↓+4NH3→2[Ag(NH3)2]++CrO2−4
( C)Cr3++3OH−→Cr(OH)⏐↓ (green).
Cr(OH)3+OH−→[Cr(OH)4]−(green coloured solution complex.)
(D)3+4SO2++7Cr2O2−7+2H+→2Cr3+[Y]+3+6SO2−4+H2O
|
Similar Questions
Knowledge Check
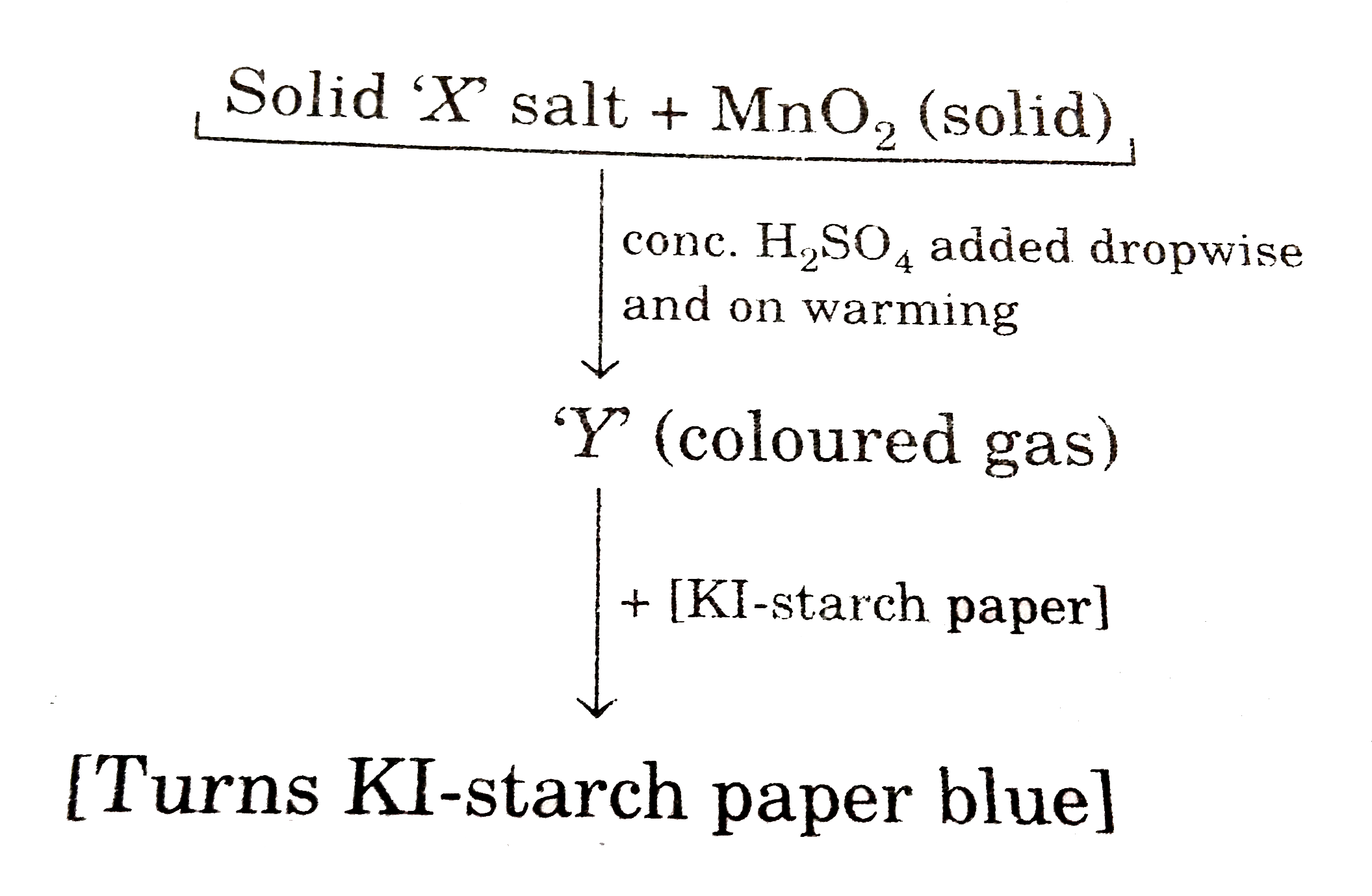
Which of the following statement(s) are correct?AMnO2 oxidizes halide ions into dihalogenBReducing nature of halide ions follow the order, F−<CI−<Br−<I−CY' gas may be I2, which turns KI-strach paper into blue colour.DY' gas may be CI2 and Br2 which oxidises KI to I2 and turns strach paper blue.Consider the reactions shown below :
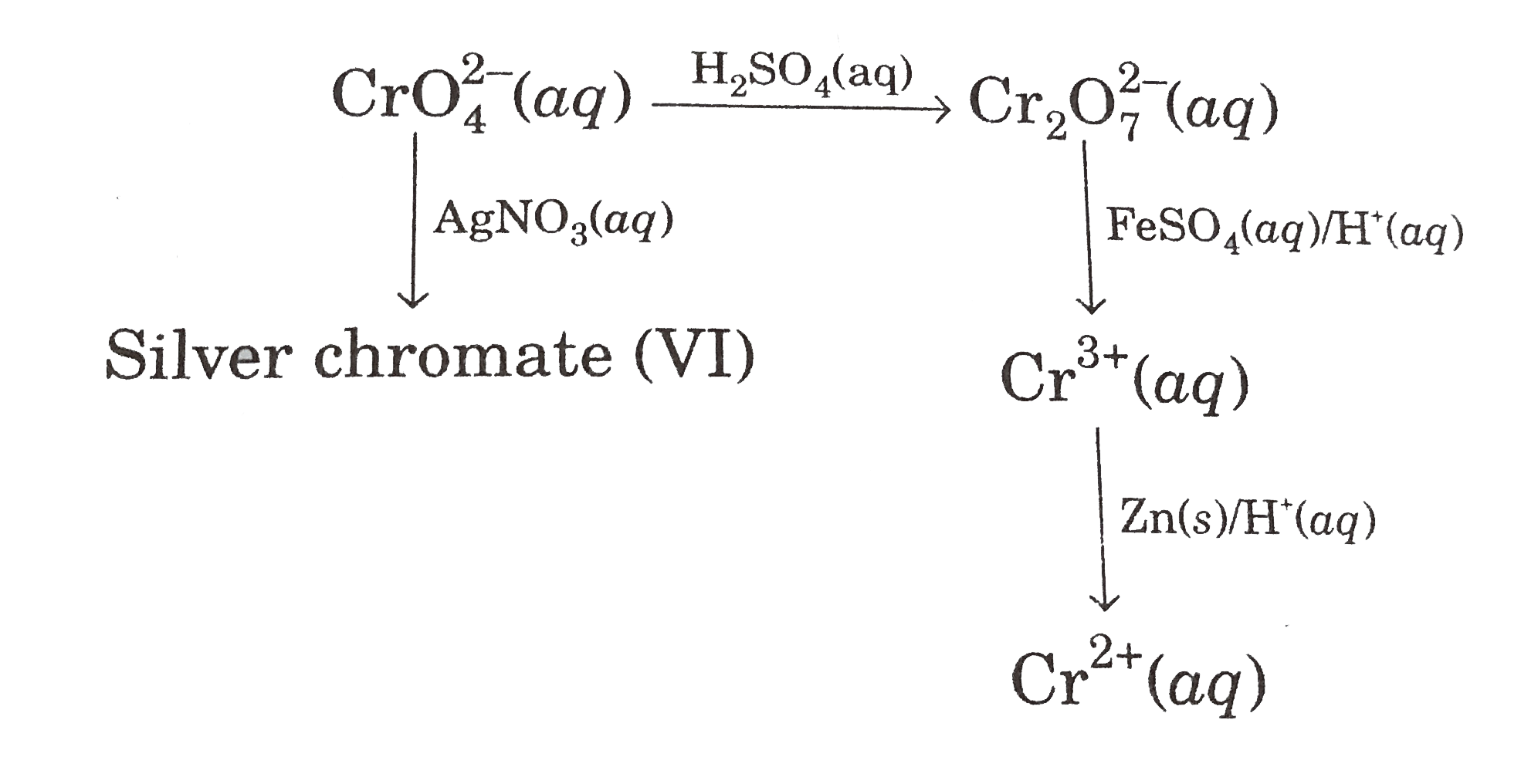
Which of the follwoing statements is false?ASilver chromate (VI) has the formula Ag2CrO4.BThe minimum mass of zinc required to reduce 0.1 mole of Cr3+toCr2+ is 6.54gCThe conversion of CrO2−4 into Cr2O2−7 is not a redox reaction .DThe equation Cr2O2−7+14H+6Fe2+→6Fe3++2Cr3++7H2O correctly describes the reduction of Cr2O2−7 by acidified FeSO4.Which of the following statements/reactions are correct ?
A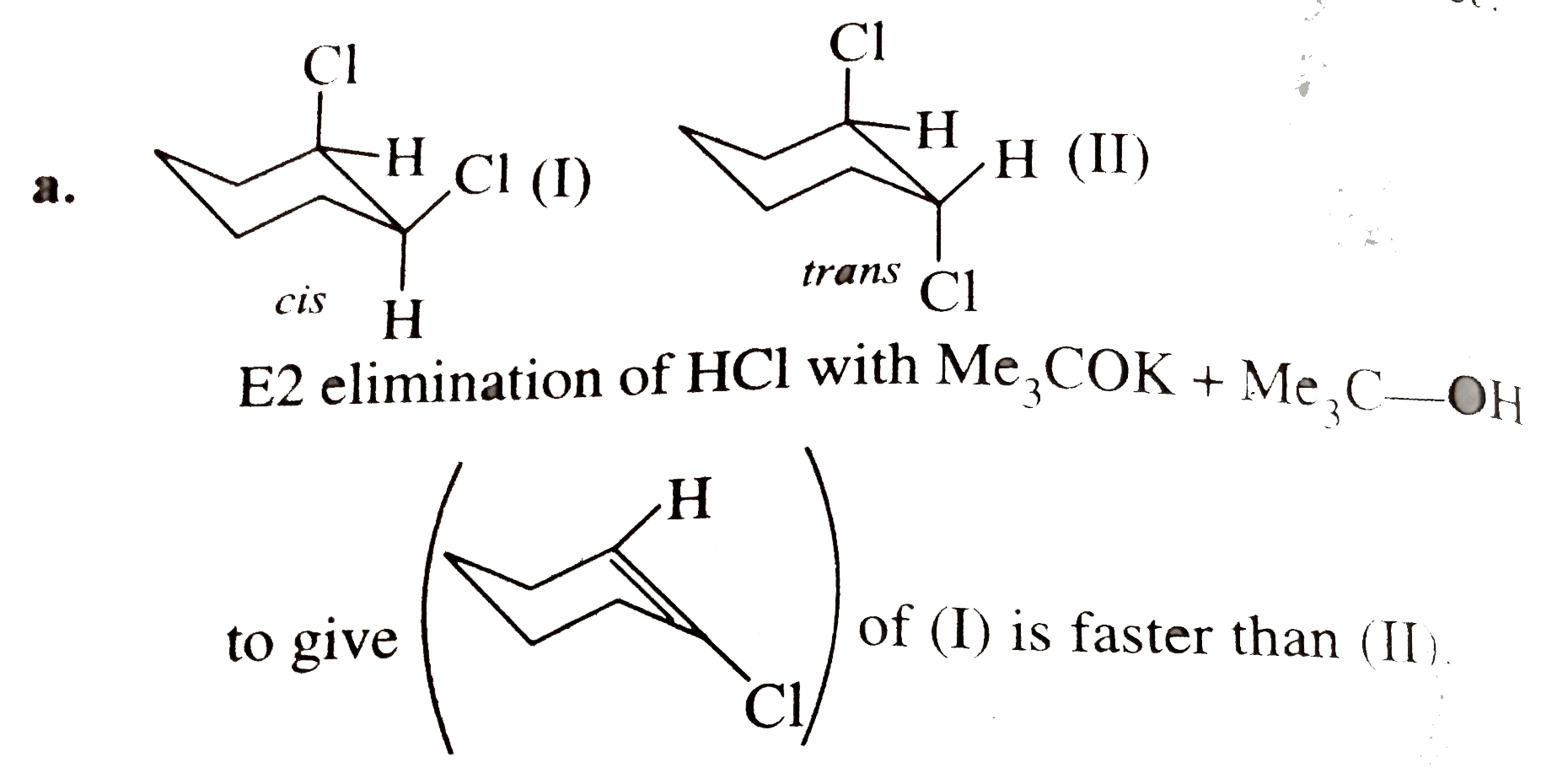 B
B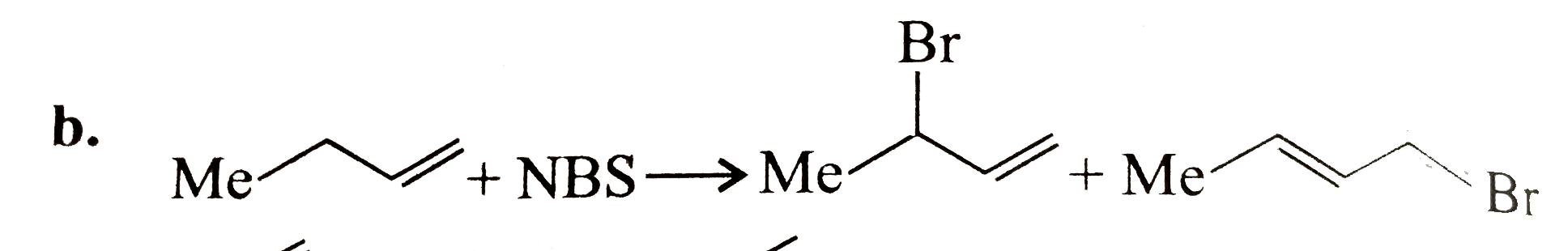 C
C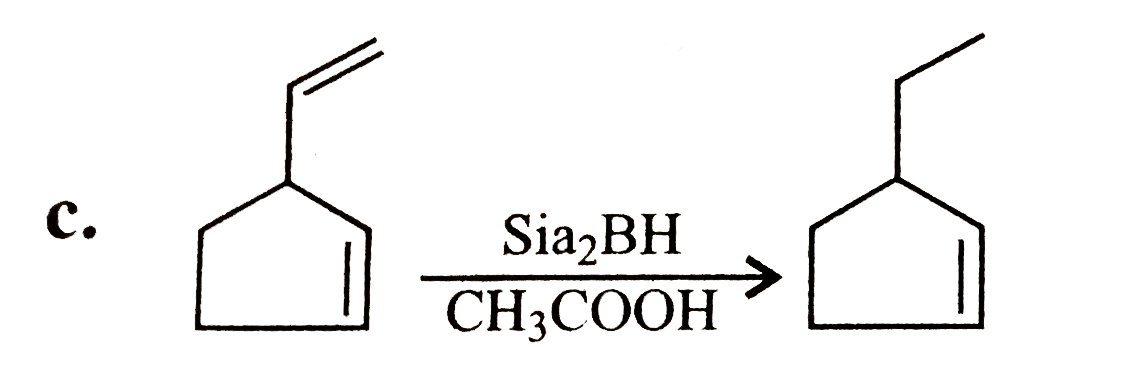 D
D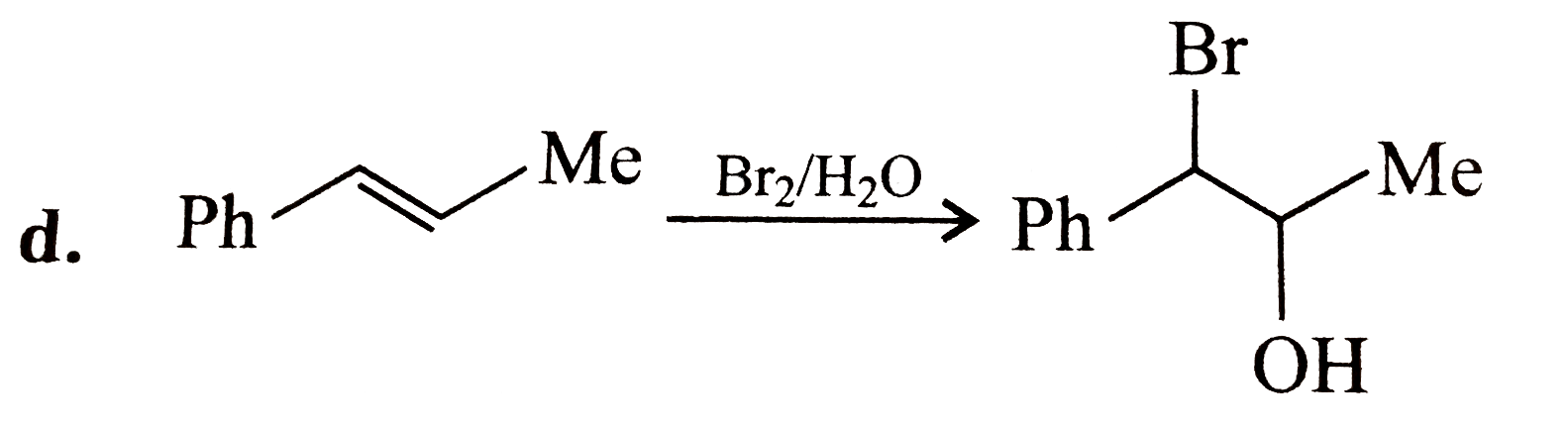
Which of the following statements/reactions are correct ?
View SolutionWhich of the following reaction(s) is/are correct?
View SolutionIn the reaction given below :
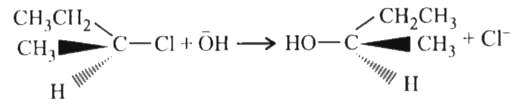
Which of the following statements is/are incorrect ?
View SolutionWhich of the following statement are correct reactions?
View SolutionConsider the reaction sequence given below :
Which of the following statements is true :
View Solution
