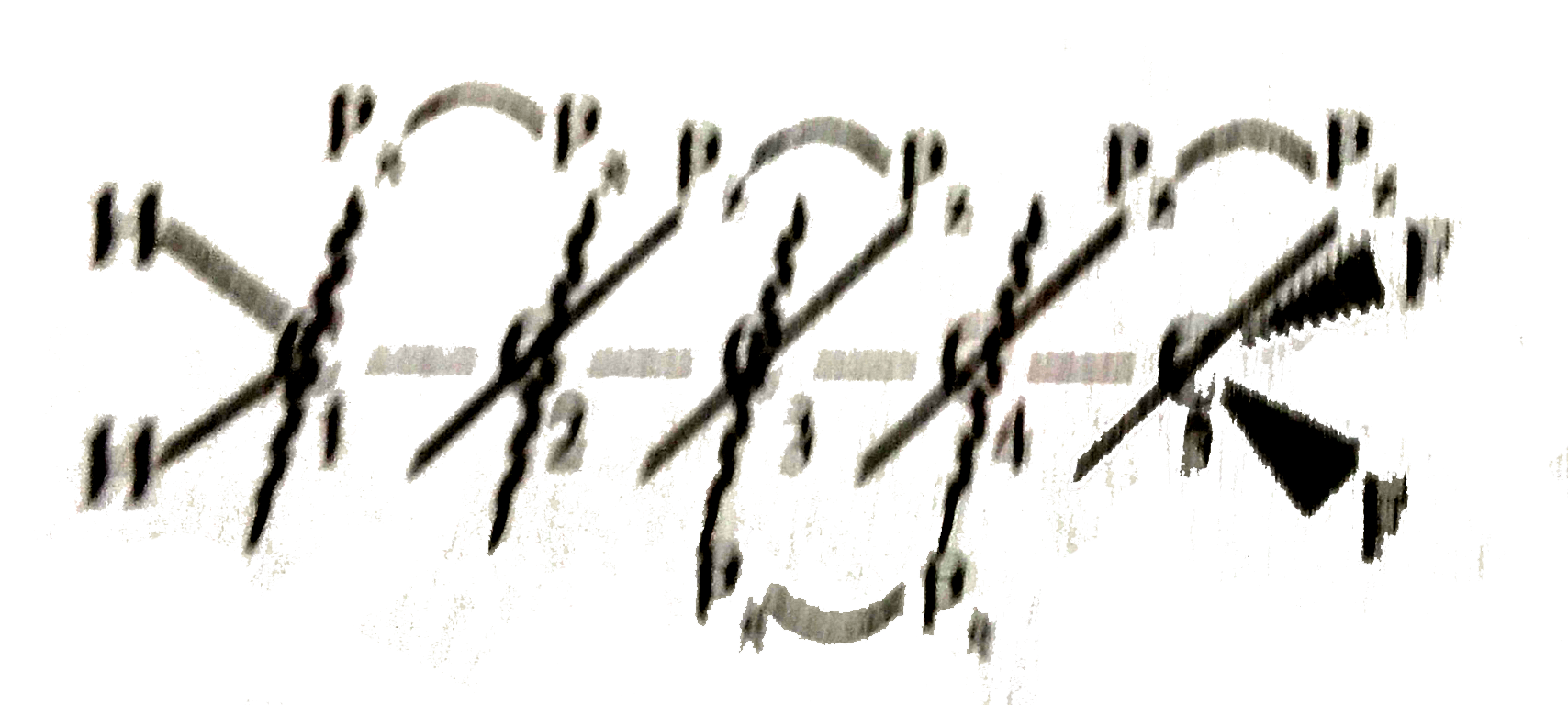Select the correct statement regarding below figure if in this figure C1 has sp2(S+Py+Pz) hybridisation and C4 has sp(SPx hybridisation.
H2C1=C2=C3=C4=F52
A
Over all molecule is planar
B
Nodel plane of π - bond of C2−C3 atoms is present is
C
Nodel plane of π- bond of C3−C4 atoms is present in YZ plane
D
Tota nine sigma bonds are present.
Text Solution
The correct Answer is:C
|
Topper's Solved these Questions
- TEST PAPERSBook:BANSALChapter:TEST PAPERSExercise:CHEMISTRY PART (A)
Explore 14 Videos
- TEST PAPERSBook:BANSALChapter:TEST PAPERSExercise:CHEMISTRY PART (B)
Explore 1 Video
- TEST PAPERSBook:BANSALChapter:TEST PAPERSExercise:Chemistry PART D
Explore 4 Videos
- SOLUTIONSBook:BANSALChapter:SOLUTIONSExercise:Exercise4
Explore 32 Videos
- THERMOCHEMISTRYBook:BANSALChapter:THERMOCHEMISTRYExercise:Exercise 4
Explore 15 Videos
Similar Questions
Knowledge Check
The correct name of CH3CH2−C∣∣O−C∣CNH−CHO is
A2-cyano-3-oxopentanalB2-formyl-3-oxopentanenitrileC2-cyano-1,3-pentadieneD1,3-dioxo-2-cyanopentaneThe correct name of CH3CH2−C∣∣O−C∣CNH−CHO is
A2-cyano-3-oxopentanalB2-formyl-3-oxopentanenitrileC2-cyano-1,3-pentadieneD1,3-dioxo-2-cyanopentaneThe correct IUPAC name of
CH3−C∣CH3=CH∣C2H5A1,2 -diethyl buteneB2 - ethyl -3- methyl penteneC3 , 4 - dimethyl hex -3- eneD2 - methyl pent -2- ene
Give its IUPAC name.
CH3−C∣C2H5H−CH2−C∣C2H5H−CH3
View SolutionThe IUPAC name of :
H5C2−C∣CHOH−C∣CH3H−C2H5 is
View SolutionThe correct IUPAC name
CH3−C∣CH3=C∣C2H5−CH3
View SolutionIUPAC name of : CH3−CH2−C∣∣O−CH∣CH3−C∣∣O−C2H5
View SolutionThe IUPAC name of:
CH3−C∣Cl=C∣CH3−−CH∣C2H5−CH2C≡CH is
View Solution