Consider the following statements:
(I) Li+(aq) is greater in size than Cs+(aq)
(II) H−F is more thermal stable than HI.
maleic acid is more acidic than fumaric acid.
The correct statements are -
A
I and III
B
II alone
C
I and II
D
I, II and III
Video Solution
Text Solution
The correct Answer is:D
(i) Size in aqueous medium ∝ hydration energy
hydration energy ∝1size of cation
(ii) H−F H−1
1s−2p 1s−5p
strength order 1s−2p>1s−5p so thermal stablity of HF>HI
|
Topper's Solved these Questions
- TEST PAPERSBook:BANSALChapter:TEST PAPERSExercise:CHEMISTRY PART (A)
Explore 14 Videos
- TEST PAPERSBook:BANSALChapter:TEST PAPERSExercise:CHEMISTRY PART (B)
Explore 1 Video
- TEST PAPERSBook:BANSALChapter:TEST PAPERSExercise:Chemistry PART D
Explore 4 Videos
- SOLUTIONSBook:BANSALChapter:SOLUTIONSExercise:Exercise4
Explore 32 Videos
- THERMOCHEMISTRYBook:BANSALChapter:THERMOCHEMISTRYExercise:Exercise 4
Explore 15 Videos
Similar Questions
Knowledge Check
Formic acid is more acidic than
ABenzoic acidBAcetic acidCPhenolDBenzene sulphoric acidPhenol is more acidic than
A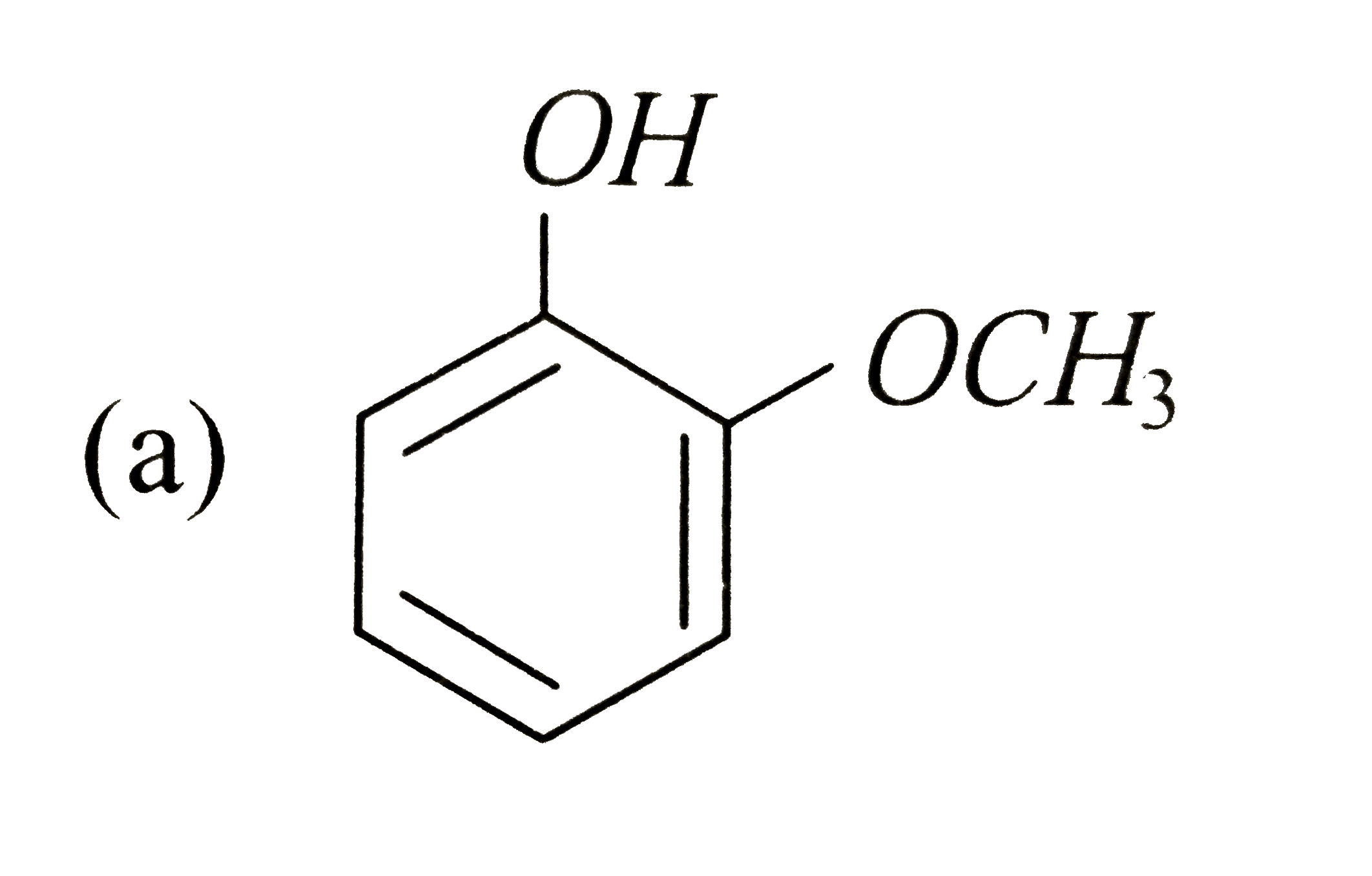 B
B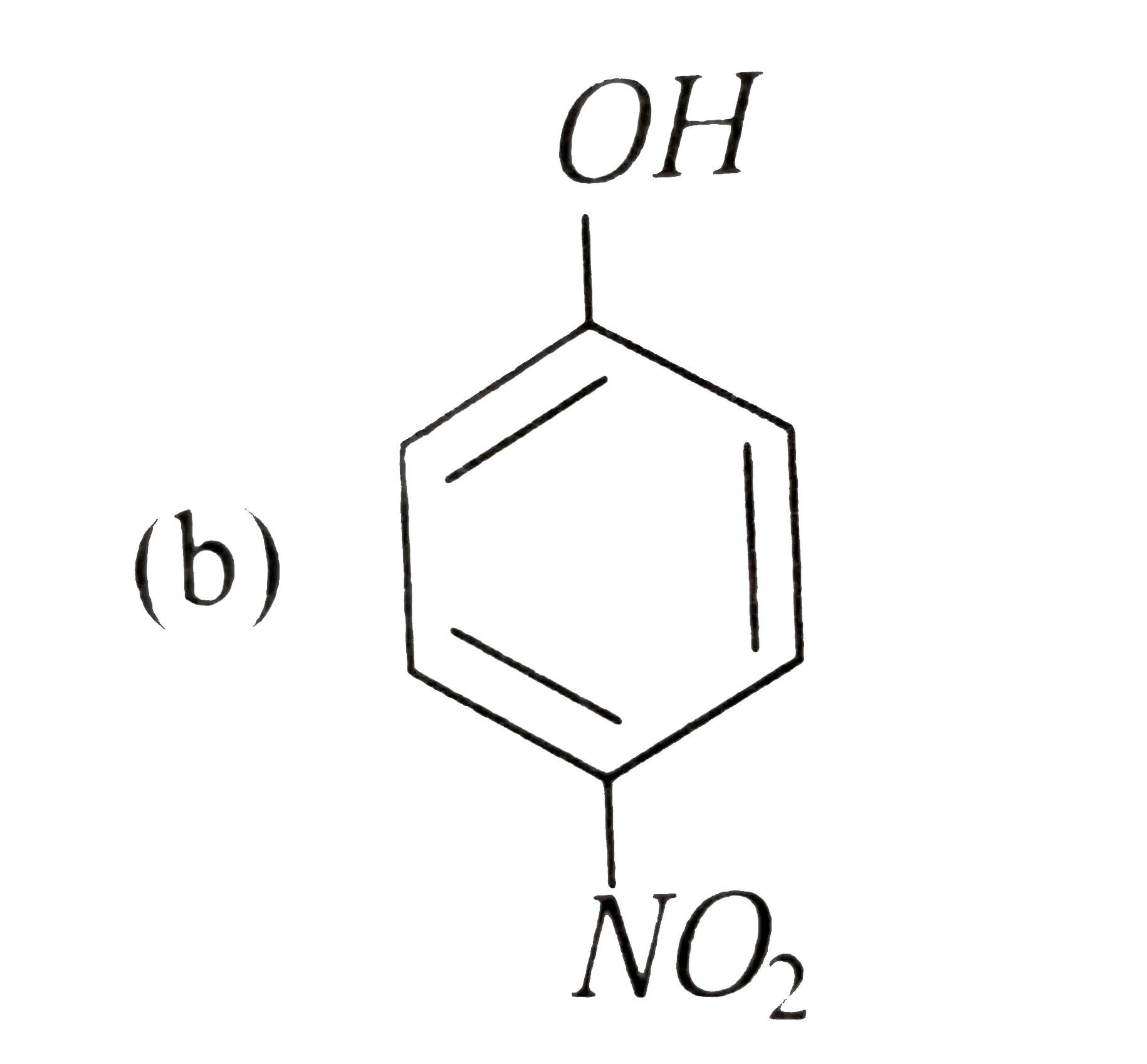 CC2H2DBoth (a) and (c)
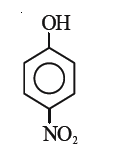
CC2H2DBoth (a) and (c)Phenol is more acidic than :
AC2H5OHBCH3COOHC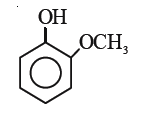 D
D