In which of the following product will be aldehyde
A
(CH3)2C=C(CH3)203−−−→Zn/H2
B
CH3CH=CH2CO/H2−−−−−−−−−−→Δ,[CO(CO)8]
C
CH3CH=CH2Pdcl2,H2O−−−−−−→CuCl2
D
CH3−C≡CHHgSO4−−−−−−−→H2SO4/H2O
Text Solution
The correct Answer is:2
Oxo process or hydroformylation of alkene. Alkene with water gas at high T & P in the presence of cobalt carbonyl forms aldehyde.
|
Topper's Solved these Questions
- CARBONYL COMPOUNDSBOOK - NARAYNACHAPTER - CARBONYL COMPOUNDSEXERCISE - STATEMENT TYPE
24 Videos
- CARBONYL COMPOUNDSBOOK - NARAYNACHAPTER - CARBONYL COMPOUNDSEXERCISE - LEVEL-IV
37 Videos
- CARBONYL COMPOUNDSBOOK - NARAYNACHAPTER - CARBONYL COMPOUNDSEXERCISE - LEVEL-II(C.W)
30 Videos
- BENZENEBOOK - NARAYNACHAPTER - BENZENEEXERCISE - Level-6
121 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - NARAYNACHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - EXERCISE -4
54 Videos
Similar Questions
Knowledge Check
Which of the following reaction will aldehyde?
A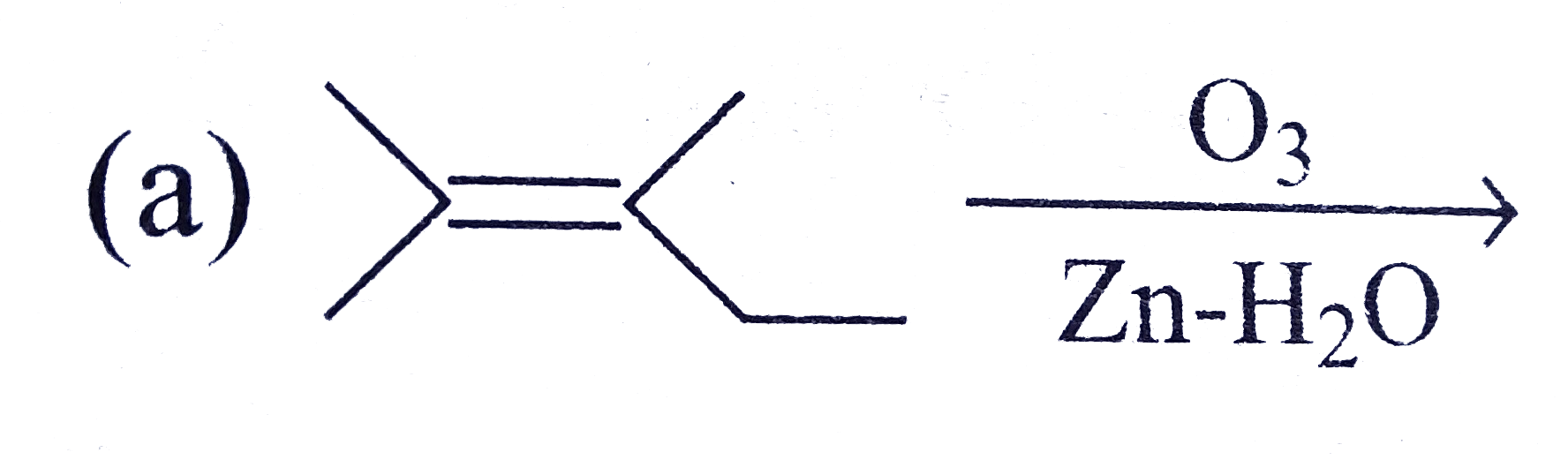 B
B C
C D
D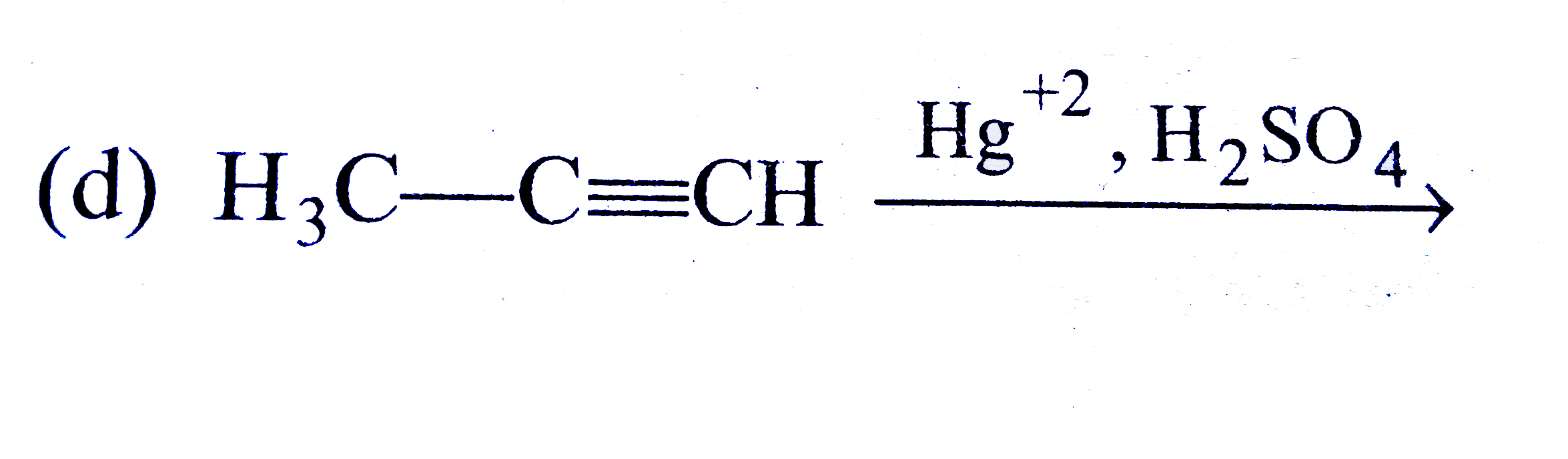
In which of the following reactions aldehydes and ketones are ditinguished:
AReaction with phenyl hydrazineBReaction with hydroxylamineCReaction with semicarbazideDReaction with silver nitrate mixed with ammoniaWhich of the following reactions will produce aldehyde?
A BH3C−CH=CH2CO+H2−−−−−−−→CO2(CO)8CH_(3)C-C-=CHunderset(underset(ϴ)(O)H//H_(2)O)(to)DH3C−C−o=ChHg+2,H2SO4−−−−−−−−→
BH3C−CH=CH2CO+H2−−−−−−−→CO2(CO)8CH_(3)C-C-=CHunderset(underset(ϴ)(O)H//H_(2)O)(to)DH3C−C−o=ChHg+2,H2SO4−−−−−−−−→
Which of the following will give an aldehyde on oxidation ?
View SolutionWhich of the following will react with aldehydes, ketones and also with nitrosobenzene ?
View SolutionA diene, buta - 1, 3 - diene was subjected to ozonolysis to prepare aldehydes. Which of the following aldehydes will be obtained during the reaction ?
View SolutionA diene, buta - 1, 3 - diene was subjected to ozonolysis to prepare aldehydes. Which of the following aldehydes will be obtained during the reaction ?
View SolutionWhich of the following reaction will give β-keto aldehyde as the final product ?
View Solution

