The correct order of increasing acidic strength is
A
Phenol ltEthanol lt Chloroacetic acid lt Acetic acid
B
Ethanol lt Phenol lt Chloroacetic acid lt Acetic acid
C
Ethanol lt Phenol lt Acetic acid lt Chloroacetic acid
D
Chloroacetic acid lt Acetic acid lt Phenol lt Ethanol
Video Solution
Text Solution
The correct Answer is:3
Inductive effect
|
Similar Questions
Knowledge Check
The correct order of increasing acidic strength of the following acids is
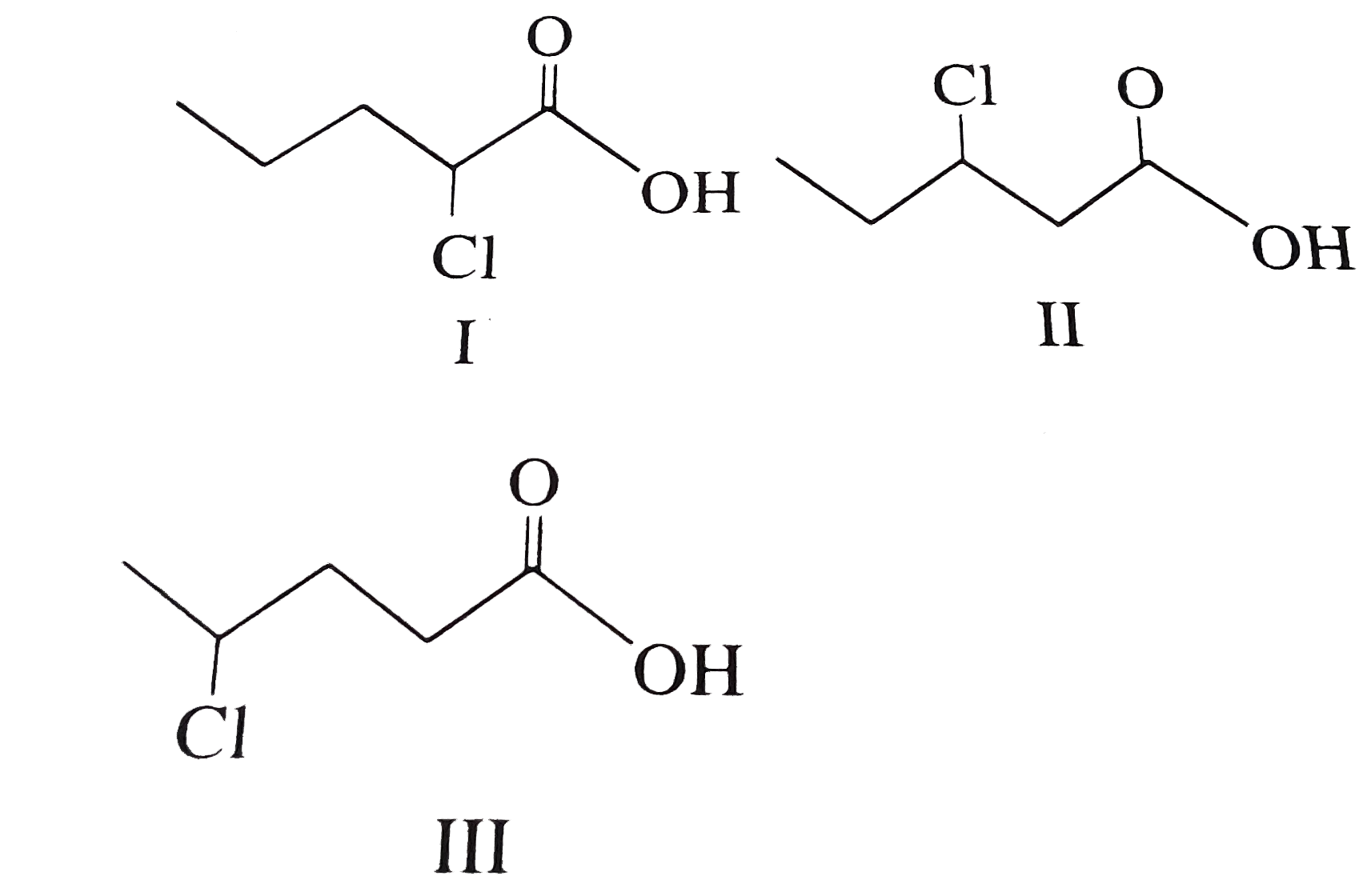 AI<II<IIIBI<III<IICIII<II<IDII<III<I
AI<II<IIIBI<III<IICIII<II<IDII<III<IThe correct order of increasing acidic strength of the following acids is
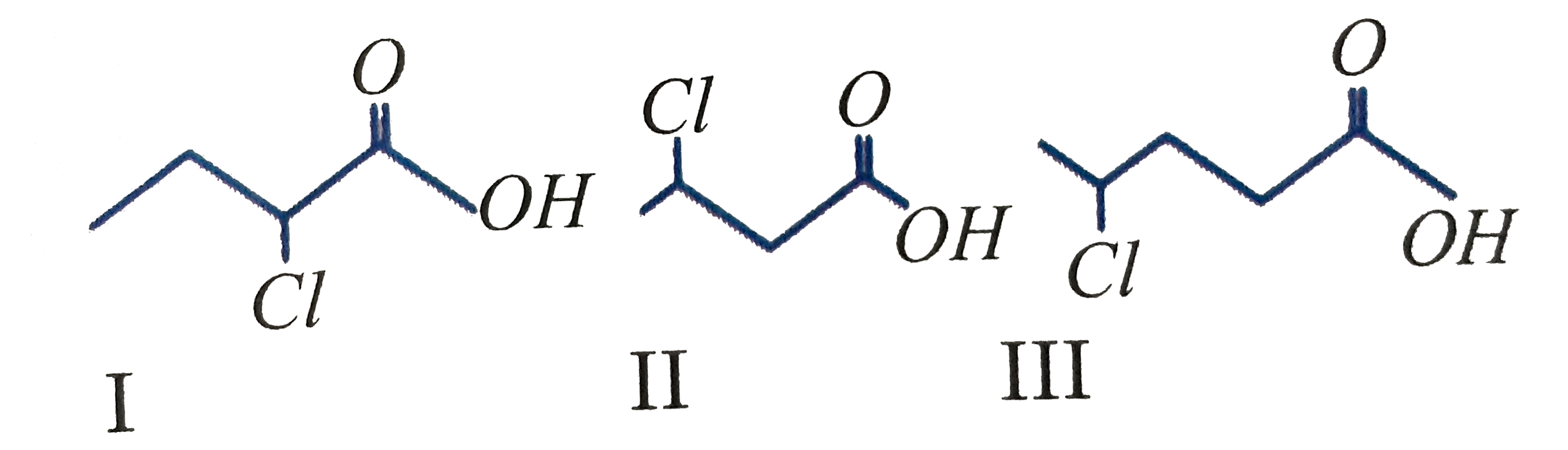 AI<II<IIIBI<III<IICIII<II<IDII<III<I
AI<II<IIIBI<III<IICIII<II<IDII<III<IAmong

The correct order of increasing acid strength is:AIltIIIltIIlt IVBIltIIltIIIltIVCIIltIltIIIltIVDIIltIIIltIltIV
Among ethanol (I), acetic acid (II), phenol (III) and benzoic acid (IV), the correct order of increasing acid strength is
View SolutionAmong ethabol (I) Acetic acid (II) phenol (III) and Benzoic acid (IV) the correct order of increasing acid strength is
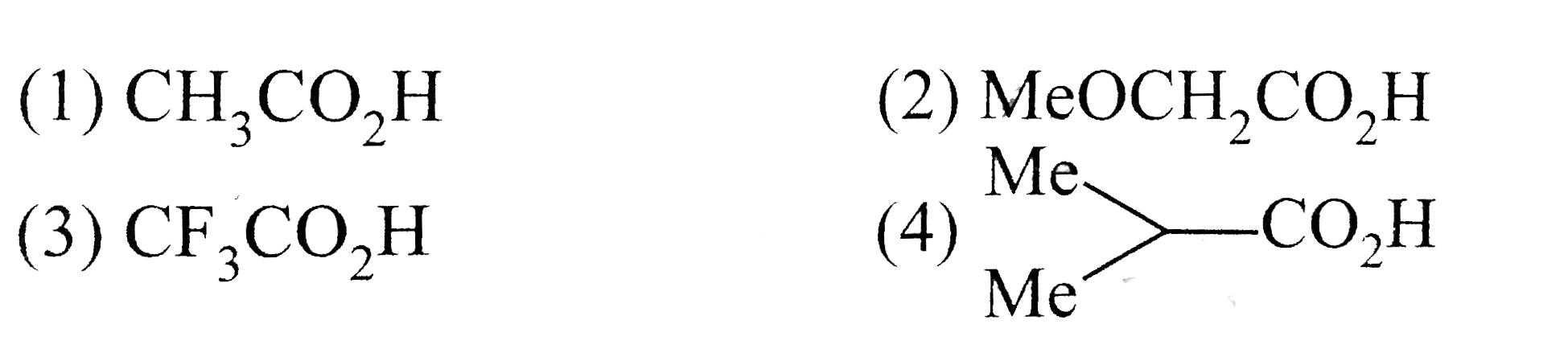
View SolutionThe correct order of increasing acid strength of the compounds:
View SolutionThe correct order of the increasing acidic strengths of HClO,HClO2,HClO3,HClO4 is
View SolutionThe correct order of increasing acid strength of the compounds
(a) CH3COOH
(b) MeO−CH2CO2H
(c) CF3CO2H
(d)
View Solution

