For the following data answer the questions :
Reaction : A+B → P
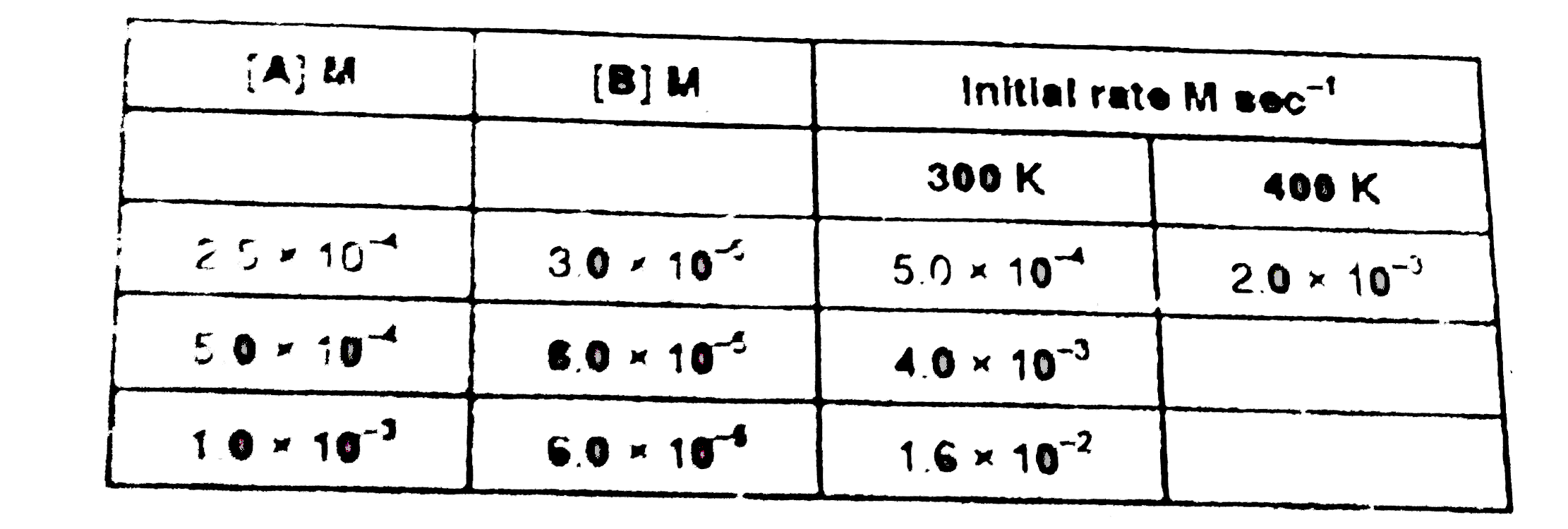
The value of rate constant at 300 K is (M−2sec−1):
2.667×108
2.667×105
2.667×106
2.667×109
The correct Answer is:A
k300=5×10−4(2.5×10−4)2(3×10−5)=2.677×108
Similar Questions
The rate constants of a reaction at 500 k and 700 k are 0.02"sec"^(-1) and 0.07 "sec"^(-1) respectively. Calculate the value of E_(a)
View SolutionThe rate constants of a reaction at 300K and 280 K respectively are K1 and K2. Then
View SolutionFor the reaction : A+2B→P the rate law is given by rate =k[A][B]2
From the following data , calculate x and y .
 .
.
View SolutionFor the decomposition of azoisopropane to hexane and nitrogen at 543 K, the following data are obtained. At o sec P (mm of Hg) is 35 at 360 sec P is 54 at 720 sec P is 63 Calculate the rate constant.
View SolutionFor the reaction : 2H_(2) +2NO hArr 2H_(2)O +N_(2) , the following rate data was obtained : Calculate the following: (1) The overall order of reaction. (2) The rate law. (3) The value of rate constant (k).
View SolutionThe rate constants ofa reaction at 500 K and 700 K are 0.025sec−1 and 0.075sec−1 respectively. Calculate the energy ofactivation of the reaction. (R=8.314JK−1 and log 3 = 0.447)
View SolutionIdentify the order of reaction from each of the following units of rate constant (k) : (a) "mole" L^(-1) sec^(-1) (b) Mol^(-1) sec^(-1)
View SolutionThe following data pertains to reaction between A and B.

Which of the following inference(s) can be drawn from the above data?
1.Rate constant of the reaction is 1.0×10−4
2.Rate law of the reaction is, rate = k[A] [B].
3.Rate of reaction increases four times on doubling the concentration of both the reactants.
View Solution

