For the reaction
2NO→2NOCl
at 300 K, following data are obtained:
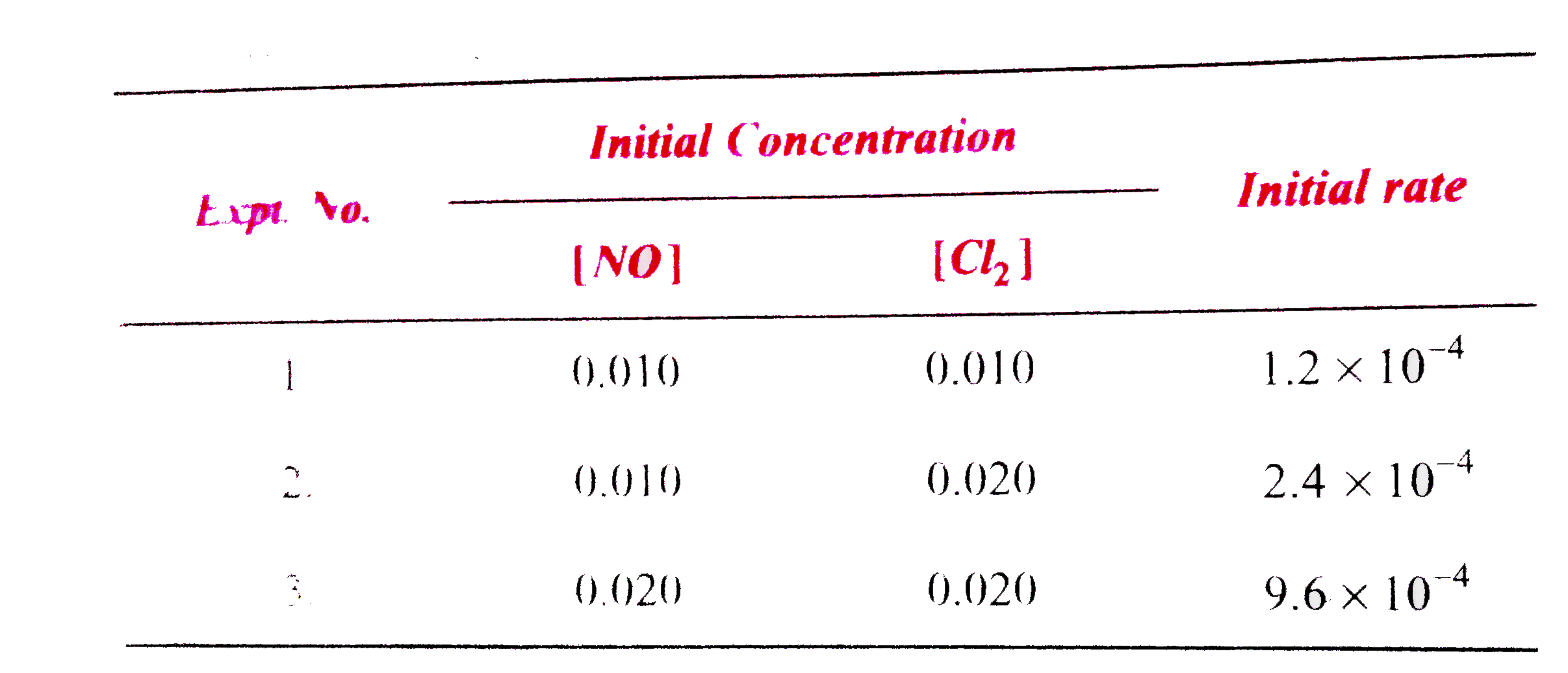
Write rat law for the reaction. What is the order of the reaction? Also calculate the specific rate constant.
Step by step video & image solution for For the reaction 2NOrarr2NOCl at 300 K, following data are obtained: Write rat law for the reaction. What is the order of the reaction? Also calculate the specific rate constant. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL KINETICSBook:OP TANDONChapter:CHEMICAL KINETICSExercise:ILLUSTRATIONS
Explore 34 Videos
- CHEMICAL KINETICSBook:OP TANDONChapter:CHEMICAL KINETICSExercise:PRACTICE PROBLEMS
Explore 70 Videos
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:OP TANDONChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Integer
Explore 4 Videos
- Chemical Thermodynamics and ThermochemistryBook:OP TANDONChapter:Chemical Thermodynamics and ThermochemistryExercise:Self Assessment (Integer type).
Explore 3 Videos
Similar Questions
For the given reaction, A+B→ Products following data were obtained
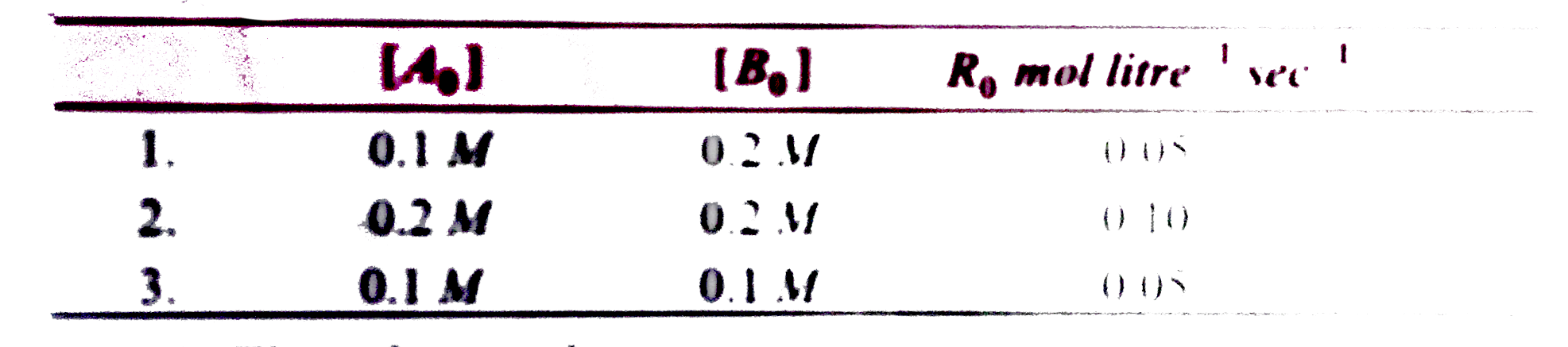
(i) Write the rate law expression.
(ii) Find the rate constant.
View SolutionFor a reaction 2P + Q → S , following data were collected. Calculate the overall order of the reaction. Also find out the reaction rate constant.
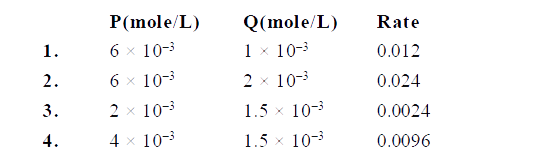
View Solution
Knowledge Check
If the rate of the reaction is equal to the rate constant , the order of the reaction is
A1B2C0D3For a creactionj, the following data were obtained:
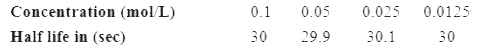
the order of reaction is:A2B1C0Dfractional
For the reaction,
2NO+2H2→N2+2H2O,
the following kinetic data were obtained.
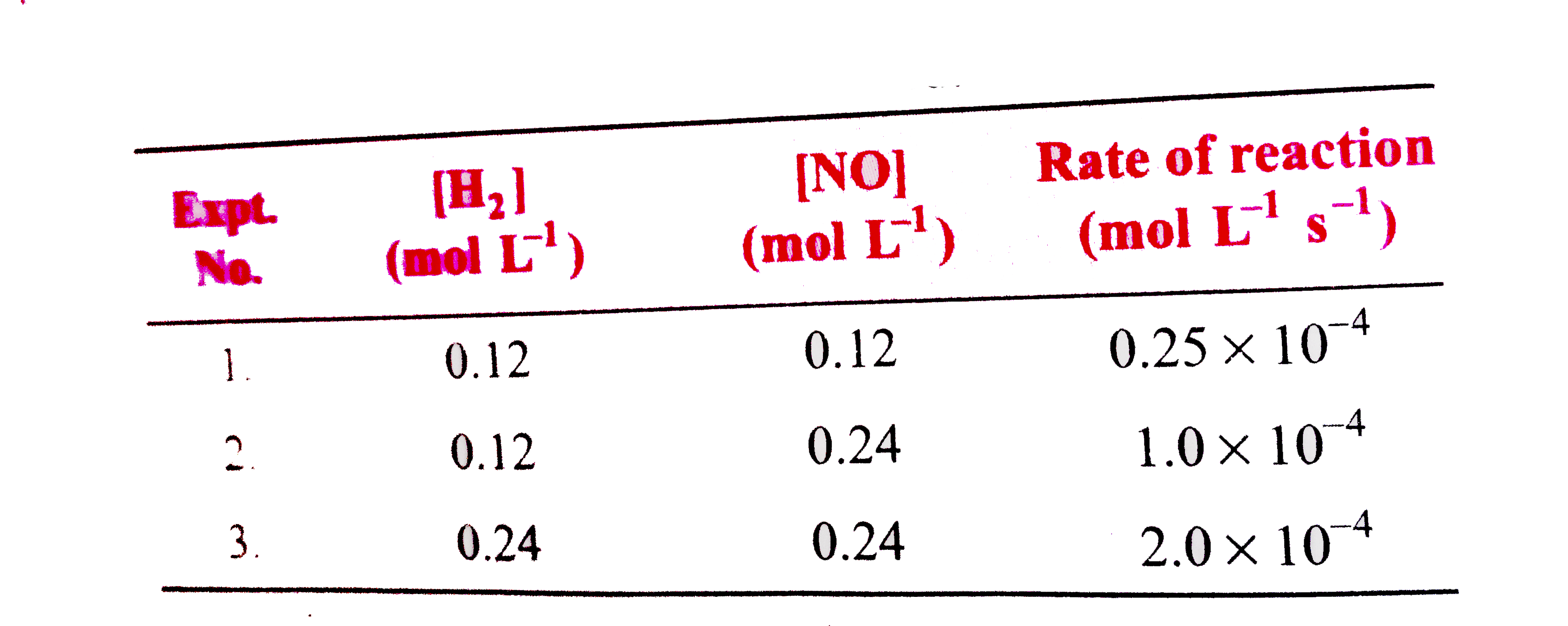
Determine the rate law and the rate constant
View SolutionFor the given reaction
A+B→ Products,
the following data are given.
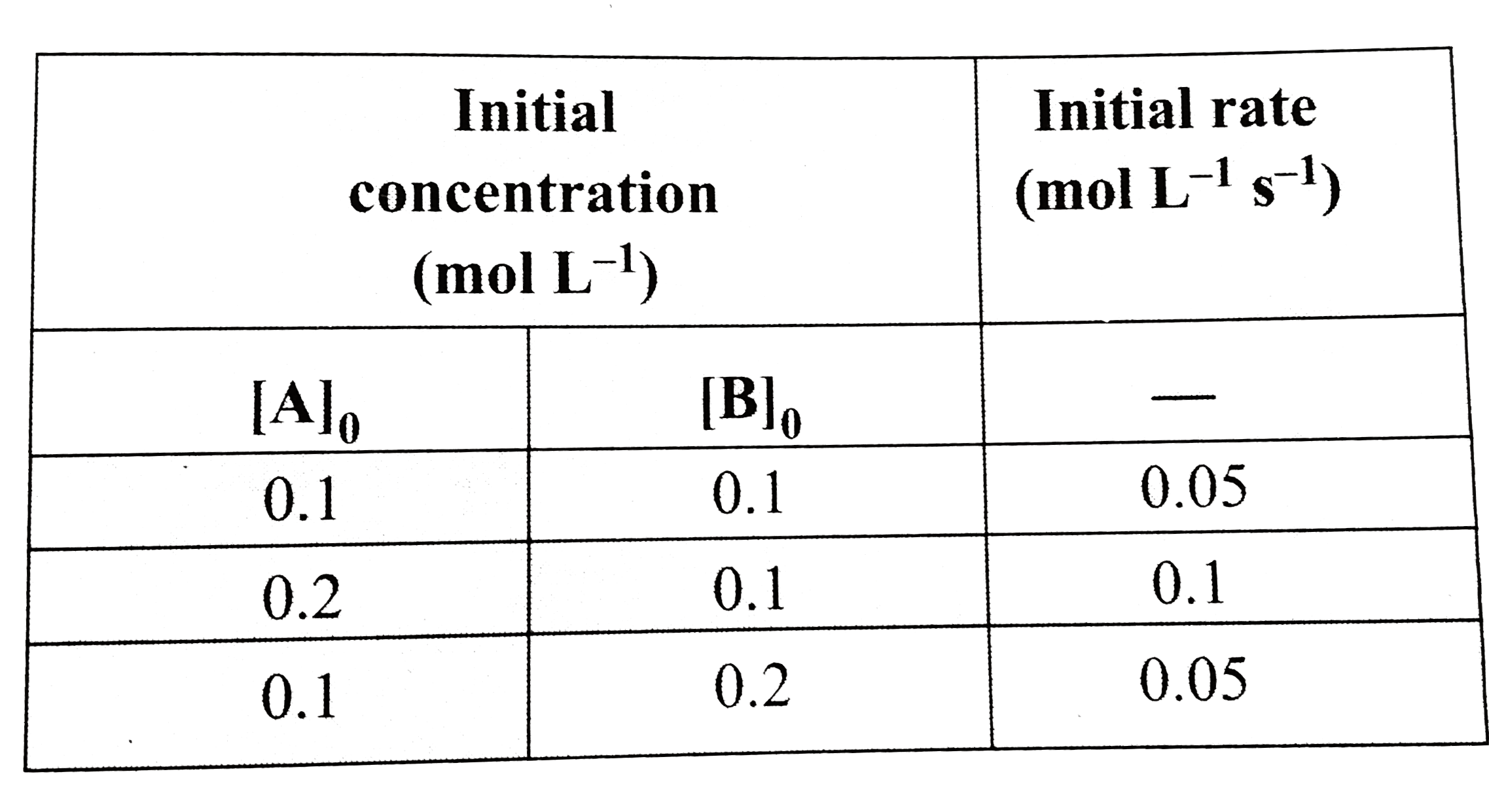
(a) Write the rate equation.
(b) Calculate the rate constant.
View SolutionThe following initial rate data were obtained at 300K for the reactions:
2A+B→C+D

Deducethe rate law.
View SolutionFrom the following data show that the decomposition of H2O2 is a reaction of first order. Also calculate the value of the rate constant.

View SolutionFor the given reaction, A+B→ Products Following data are given
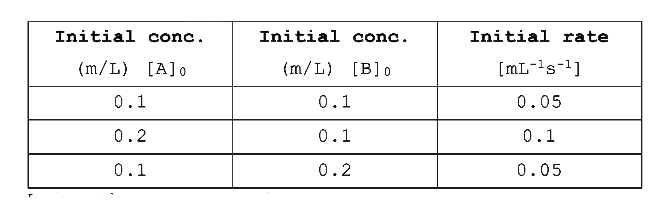
(a) Write the rate equation
(b) Calculate the rate constant
View SolutionThe following data wer obtained for a given reaction at 300 K

Calculate by what factor the rate of catalyst reaction is increases ?
View Solution
