The temperature-time graph below shows the heating curve for pure wax. From the graph answer the following :
(a) What is the physical state of the substance at the points A, B, C and D ?
(b) What is the melting point of the substance ?
(c ) What is its boiling point ?
(d) Which portions of the graph indicates that change of state is taking place.
(e ) Name the terms used for heat absorbed during change of states involved in above processes.
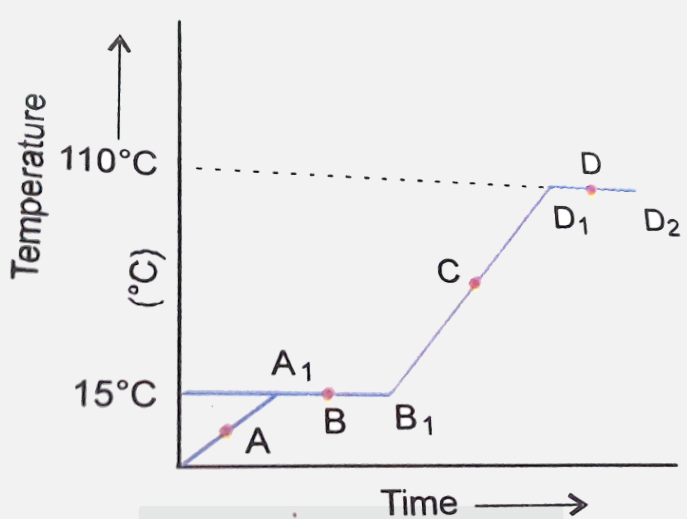
(a) At point A : Wax is in the solid state.
At point B : Wax has started melting and exists both in the solid as well as liquid states.
A point C : Wax is in liquid state.
At point D : Wax has stared boiling. Therefore, it exists both in the liquid as well as gaseous states.
(b) Melting point of wax = 15∘C
(c ) Boiling point of wax =110∘C
(d) The change of state (solid to liquid) is represented by the portion A1 to B2 (straight line).
The change of state (liquid to gas ) is represented by the portion D1 to D2 (straight line).
(e ) It is known as latent heat of fusion in case of solids and latent heat of vaporisation in case of liquids.
Similar Questions
Define 'boiling point' of a substance ? What is the boiling point of water ?
View SolutionThe given graph shows the heating curve for a pure substance. The temperature rises with time as the substance is heated :
(a) What is the physical state of the substance at the points A, B, C and D ?
What is the melting point of the substance ?
(c ) What is its boiling point ?
(d) What happens to the temperature while the substance is changing state ?
(e ) The substance is not water. How can you judge from the graph ?
View Solution
Knowledge Check
RS part of the graph indicates _________ state of substance
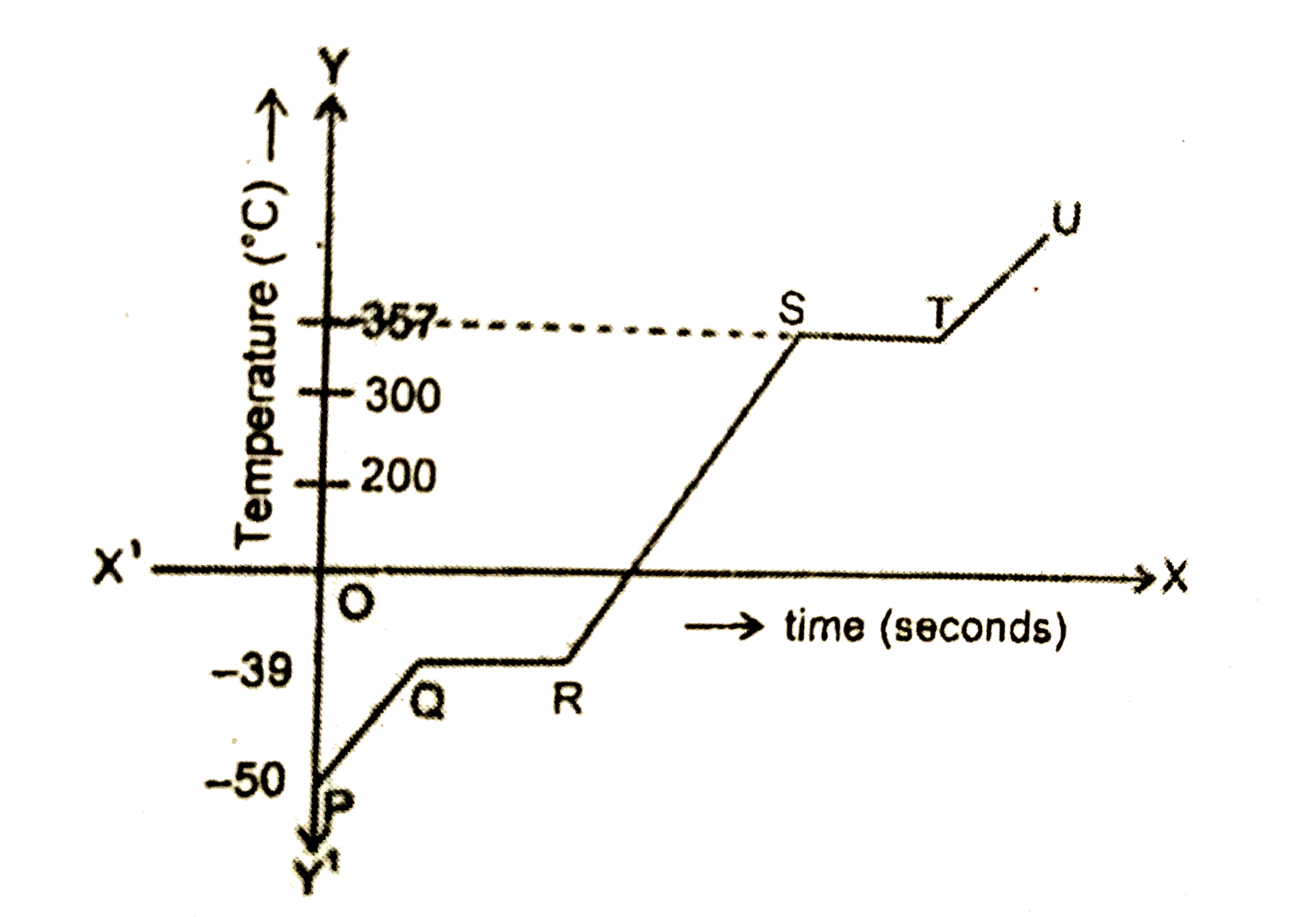 AsolidBliquidCgaseousDcannot be determined
AsolidBliquidCgaseousDcannot be determinedThe melting point of bromine is −7∘C and its boiling point is 59∘C. What is the state of bromine at room temperature
ASolidBLiquidCGasDMixture of liquid and gasIn the above graph, point D indicates
ALimiting PoointBYield pointCBreaking pointDElastic limit
The graph alogside shows the heating curve for a pure substance. The temperature rises with time as the substance is heated :
What is the physical state of the substance at the point A,B,C and D?
What is the melting point of the substance ?
What is its boiling point ?
What happens to the temperature while the substance is chaging state ?
The substace is not wter. how can you judge from the graph ?
View SolutionDefine 'melting point' of a substance ? What is the melting point of ice ?
View SolutionFrom the graph below determine the coordinates of the points A B,Cand D.
View SolutionWhy does the temperature of a substance remain constant during its melting point or boiling point ?
View SolutionIn the above graph, point B indicates
View Solution

