Bromide and nitrates both give brown fumes with conc. H2SO4. Which of the following reagent will be used to distinguish between them?
A
K2Cr2O7
B
BaCl2
C
FeSO4
D
K4.[Fe(CN)6]
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
Bromide and nitrates both give brown fumes with conc. H2SO4. Which of the following reagent will be used to distinguish between them?
AK2Cr2O7BBaCl2CFeSO4DK4[Fe(CN)6]Bromide and nitrates both give brown fumes with conc. H2SO4. Which of the following reagent will be used to distinguish between them?
AK2Cr2O7BBaCl2CFeSO4DK4[Fe(CN)6]Which of the following reagents will be useful to distinguish between
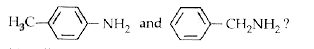 ADilute HCIBC6H5SO2ClandOH−/H2OCHONO then β-naphtolDAgNO3 in H2O
ADilute HCIBC6H5SO2ClandOH−/H2OCHONO then β-naphtolDAgNO3 in H2O
Salts which give brown vapours with conc.H2SO4 may be
View SolutionWhich of the following reagents can be used to distinguish between SO2andCO2?
View SolutionWhich of the following reagents can be used to distinguish the SO2 and CO2 ?
View SolutionMark the compound which gives carbon with conc. H2SO4
View SolutionWhich of the following reagents/tests cannot be used to distinguish allyl bromide from n-propyl bromide
View Solution

