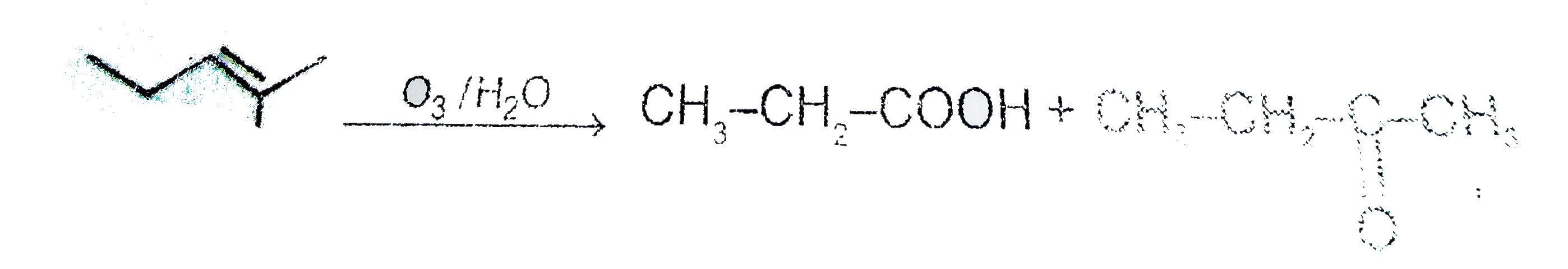On oxidative ozonolysis of 3-Methylhex-3-ene, two products A & B are formed. A gives CO2 gas with sodium bicarbonate, but B can not. The structures of A & B rae respectively :
CH3−CH2−C∣∣O−CH3−&CH3−CH2−COOH
CH3−CH2−COOH&CH3−CH2−CH=O
CH3−CH2−COOH&CH3−CH−C∣∣O−CH3
CH3−CH2−CH2−COOH&CH3−C∣∣O−CH3
The correct Answer is:C
Similar Questions
Knowledge Check
On oxidative ozonolysis of 3-Methylex-3-ene, two products A and B are formed . A gives CO2 gas with sodium bicarbonate , but B can not. The structure of A and B are respectively.
ACH3−CH2−C∣∣O−CH3&CH3−CH2−COOHBCH3−CH2−COOH&CH3−CH2−CH=OCCH3−CH2−COOH&CH3−CH2−C∣∣O−CH3DCH3−CH2−CH2−COOH&CH3−C∣∣O−CH3How many hydrocarbons having molecular mass 68 can give white precipitate with Tollen's reagent sodium bicarbonate, but B can not . The structure of A & B are respectively.
A1B2C3D4In the following reaction sequence, the products (B) to (E) are formed.
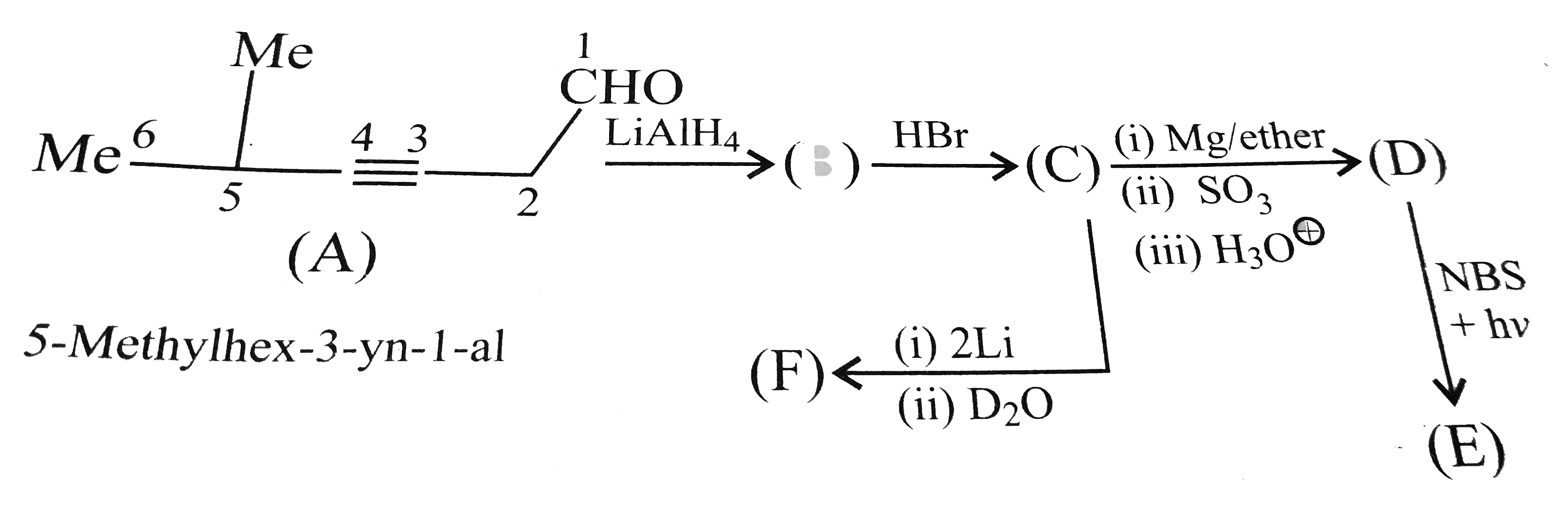 .
.
The structure of product (B) is :A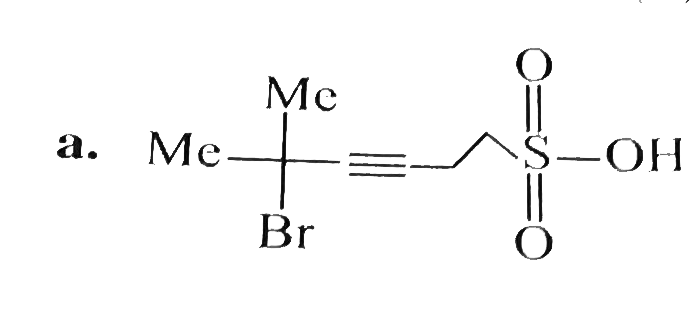 B
B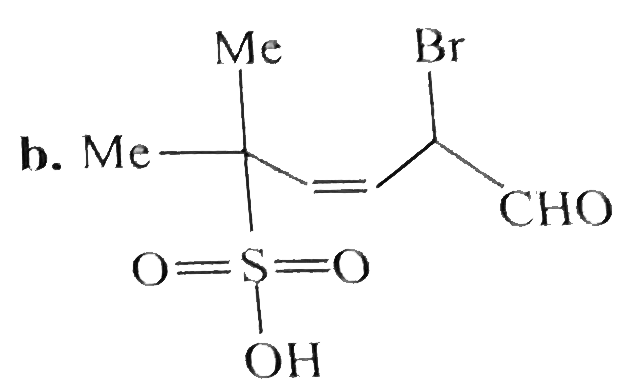 C
C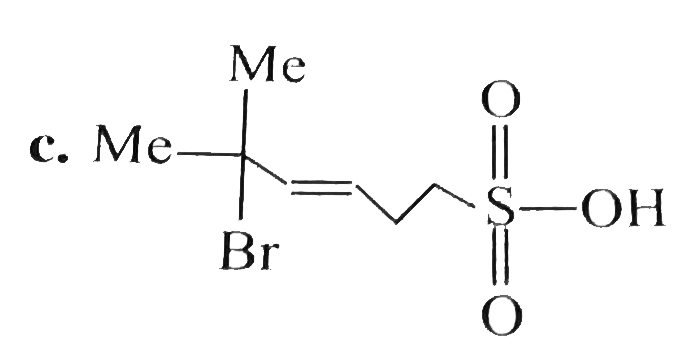 D
D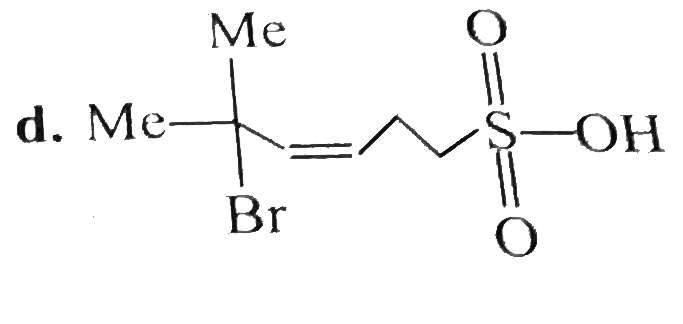
An alkene on reductive ozonolysis gives two products A and B . When these products are treated with conc. KOH one of these products gives an alcohol and an acid salt. Here the alkene can be
View SolutionAn alkene gives two moles of HCHO, one mole of CO2 and one mole of CH3COCHO on ozonolysis. The structure of alkene is
View SolutionAn alkene on reductive ozonolysis gives two products A and B . When these products are treated with conc. KOH one of these products gives an alcohol and an acid salt. Here the alkene can be
View SolutionEthanol when reacted with PCl5 gives A, POCl3 and HCl. A reacts with silver nitrite to form B (major product) and AgCl. A and B respectively are
View SolutionAn alkene (A) on ozonolysis gives (B) as one of the product, (B) can perform Cannizzaro reaction.(A) is :
View Solution