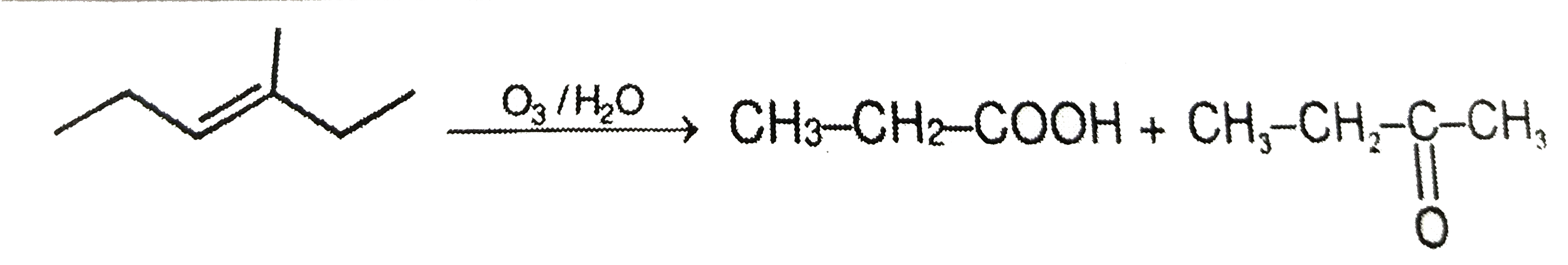On oxidative ozonolysis of 3-Methylex-3-ene, two products A and B are formed . A gives CO2 gas with sodium bicarbonate , but B can not. The structure of A and B are respectively.
CH3−CH2−C∣∣O−CH3&CH3−CH2−COOH
CH3−CH2−COOH&CH3−CH2−CH=O
CH3−CH2−COOH&CH3−CH2−C∣∣O−CH3
CH3−CH2−CH2−COOH&CH3−C∣∣O−CH3
The correct Answer is:C
Topper's Solved these Questions
Similar Questions
Heat of atomisation of NH3 and N2H4 are x kcal mol−1 and y kcal mol−1 respectively. Calculate average bond energy of N−N bond.
View SolutionCalculate the weight of sodium bicarbonate to be dissociated to give 0.56 L of CO_(2) gas.
View SolutionBut -2 -ene on ozonolysis gives
View SolutionAn alkene 3-ethylpent-2-ene will give which of the following products on ozonolysis?
View Solutionयौगिक जो जलीय सोडियम बाइकार्बोनेट विलयन द्वारा अभिक्रिया कर CO2, नहीं देता है, वह है (
View SolutionThe product formed when but - 2 - ene is on ozonolysis is ............
View SolutionHow many of the following on reductive ozonolysis will give ethanal as one of the products? 2-Methylbut-2-ene, 2-methylpropene, but-2-ene, propene, ethene, pent-2-ene, pent-3-ene, hex-3-ene
View SolutionOzonolysis products of 3,4-dimethyl hept-3-ene are
View Solution