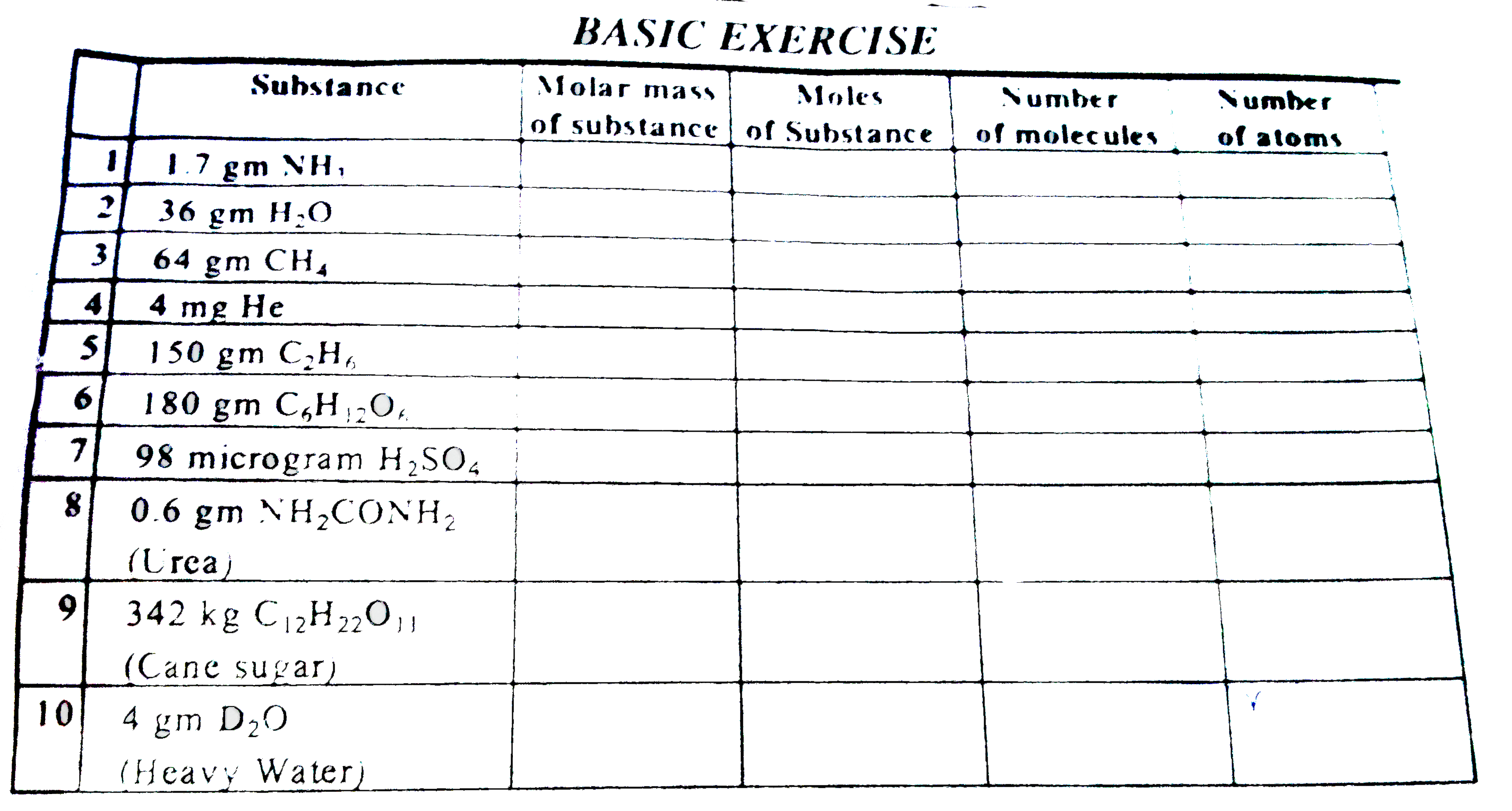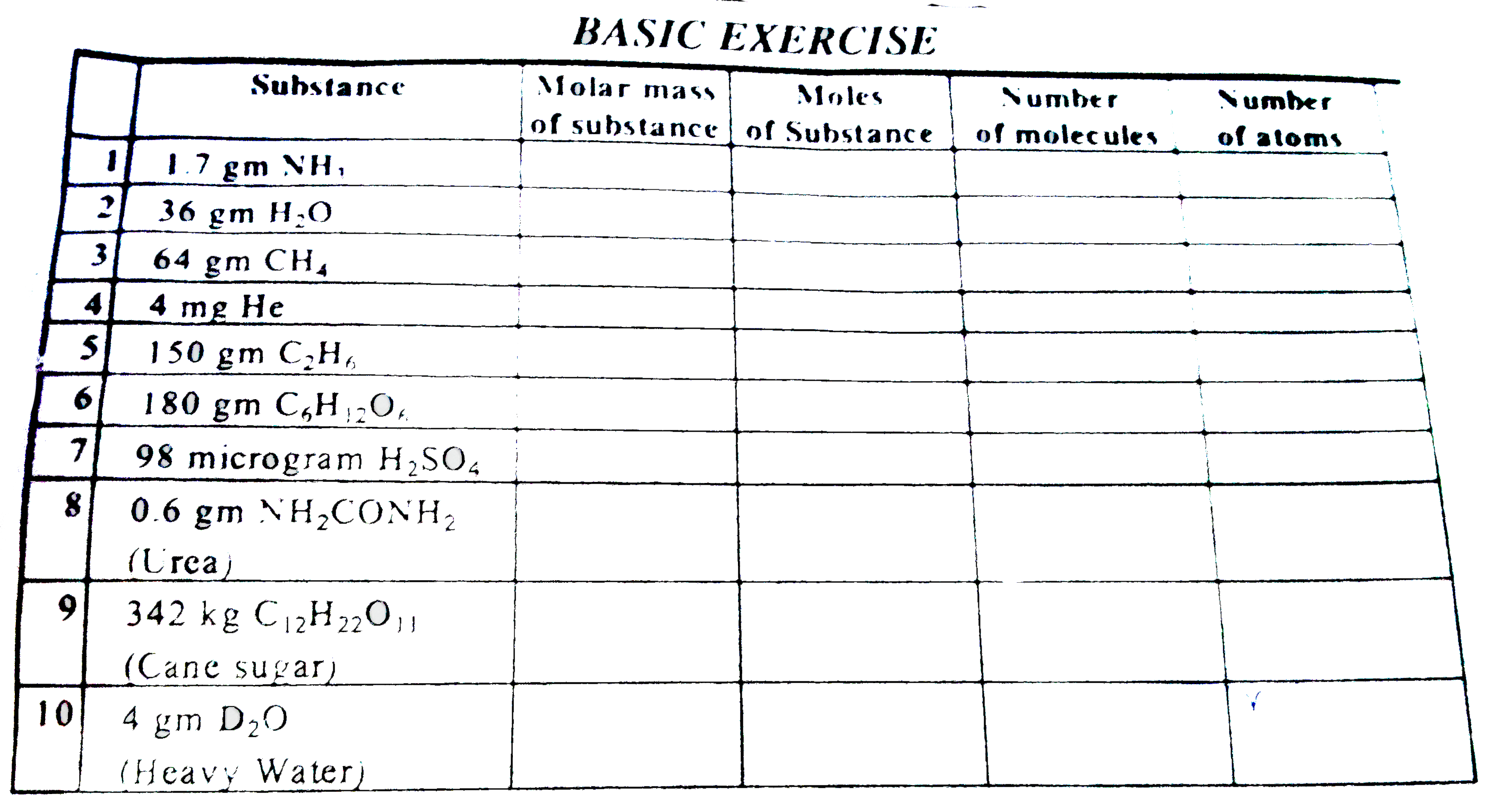
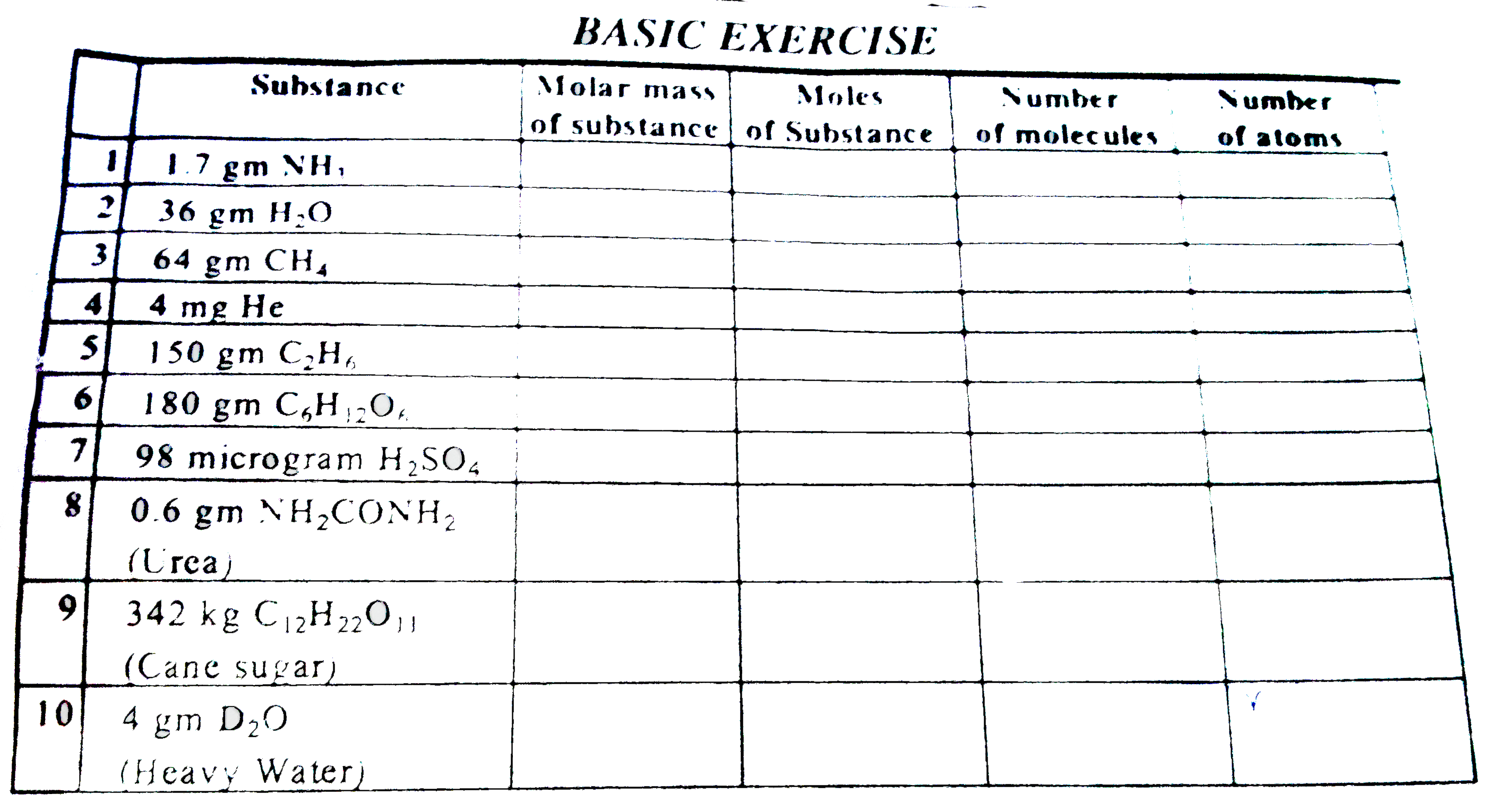
2NH3→N2+3H2
Find moles of H2 produced from 4 moles of NH3
Text Solution
The correct Answer is:(##ALNNCCHMMCE01017_A01##)
6
|
Topper's Solved these Questions
- MOLE CONCEPTBook:ALLENChapter:MOLE CONCEPTExercise:S-I (Problems Related with Different Types of Atomic Masses & Basic Concept of Mole)
Explore 53 Videos
- MOLE CONCEPTBook:ALLENChapter:MOLE CONCEPTExercise:S-II
Explore 18 Videos
- MOLE CONCEPTBook:ALLENChapter:MOLE CONCEPTExercise:Some Solved Examples
Explore 9 Videos
- METALLURGYBook:ALLENChapter:METALLURGYExercise:EXERCISE-05 [B]
Explore 26 Videos
- p-Block Element Book:ALLENChapter:p-Block Element Exercise:All Questions
Explore 20 Videos
Similar Questions
Knowledge Check
N2+3H2→NH3
AFor disproportionation reaction.BFor comproportionation reaction.CFor either intermolecular redox reaction or displacement reactionDFor either thermal combination redox reaction or thermal decomposition redox reaction.N2+3H2→NH3
AFor disproportionation reaction.BFor comproportionation reaction.CFor either intermolecular redox reaction or displacement reactionDFor either thermal combination redox reaction or thermal decomposition redox reaction.
NH3COONH4Δ−−→2NH3+CO2
If 6 moles of NH3 is produced then find moles of NH2COONH4 intially taken.
View SolutionNH2COONH4Δ−−→2NH3+CO2
If 6 moles of NH3 is produced then find moles of NH2COONH4 intially taken.
View SolutionN2+3H2→NH3
View SolutionN2+3H2→NH3
View SolutionNH3+O2→H2O+NO
how many grams of 'NO' produced, if 4moles of NH3 ,reacts with 20 moles of O2Report the answer in 10X
View SolutionCalculate the entropy of ideal mixing when 2 m oles of N2, 3 moles of H2and2 moles of NH3 are mixed at constant temperature, assuming no chemical reaction is occurring.
View Solution