A polystyrene of formula Br3C6H2(C8H8)n was prepared by heating styrene with tribromobenzyl peroxide in the absence of air. It was found to contain 10.46% bromine by weight .Find the value of n. (Br = 80)
The correct Answer is:19.≈(19)
Similar Questions
A polystyrene, having the formula Br3C6H3(C3H8)n, was prepared by heating styrene with tribromobenzoyl peroxide in the absence of air. If it was found to contain 10.46% bromine by weight, find the value of n.
View SolutionA polystryrene, having formula Br3C6H2(C8H8)n, was perpared heating styrene with tribromobenzoyl peroxide in the absence of air. If it was found to contain 10.46% bromine by weight, find the value of n.
View Solution
Knowledge Check
A polystyrene of formula Br3C6H2(C8H8)n was prepared by heating styrene with tribromobenzyl peroxide in the absence of air. It was found to contain 10% bromine by weight. Find the nearest value of n (M.W. of Br = 80) :-
A5B10C20D30A polystyrene having formula Br3C6H2(C8H8)n was prepared by heating styrene with tribromobenzoyl peroxide in absence of air. If it was found to contain 10.46% Bromine by mass, what will be the value of n.
A19B20C15D10A compound having the formula Br3C6H3(C3Hg)n contains 10.46%Br by mass, the value of n is
A54B65C45D35
A polystryrene, having formula Br3C6H2(C8H8)n, was perpared heating styrene with tribromobenzoyl peroxide in the absence of air. If it was found to contain 1.46% bromine by weight, find the value of n.
View SolutionA polystyrne having formula Br3C6H3(C3H8)n found to contain 10.46% of bromine by weight. Find the value of n. (At. wt. Br = 80)
View SolutionWrite the molecular formulae of: CH3BrandC2H5Br.
View Solution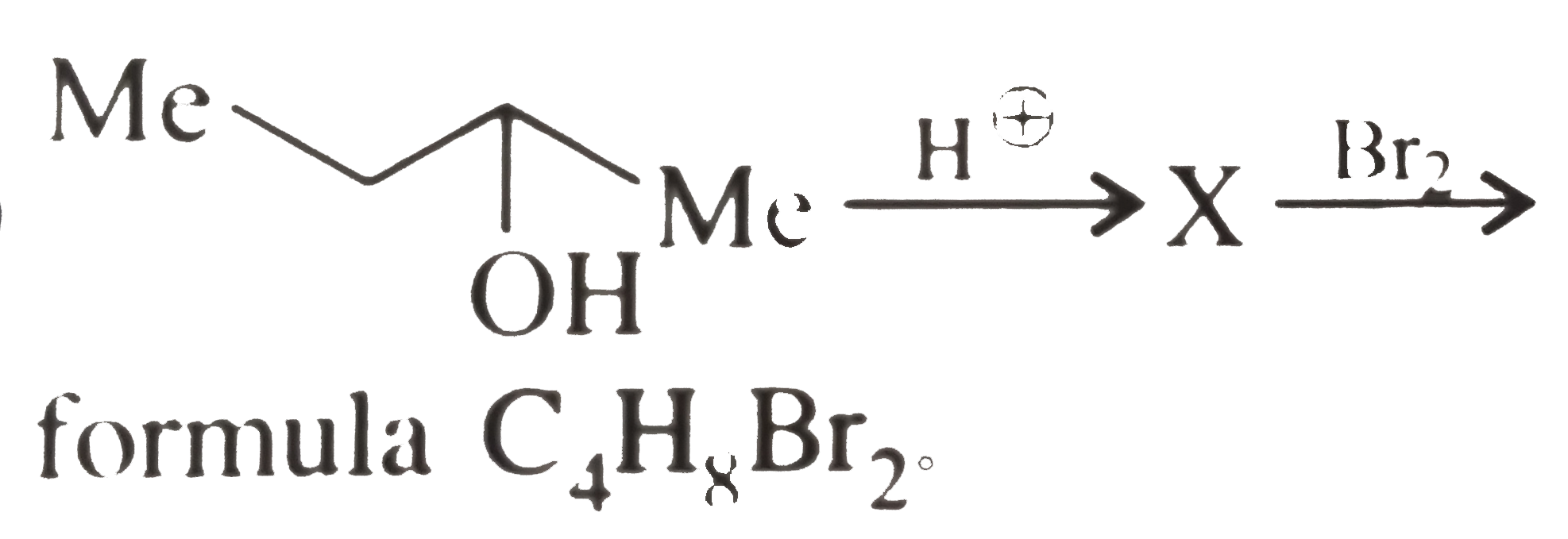
Find compound with formula C4H8Br2
How many structure of (X) are possible?
View SolutionC3H6Br2 can shown:
View Solution

