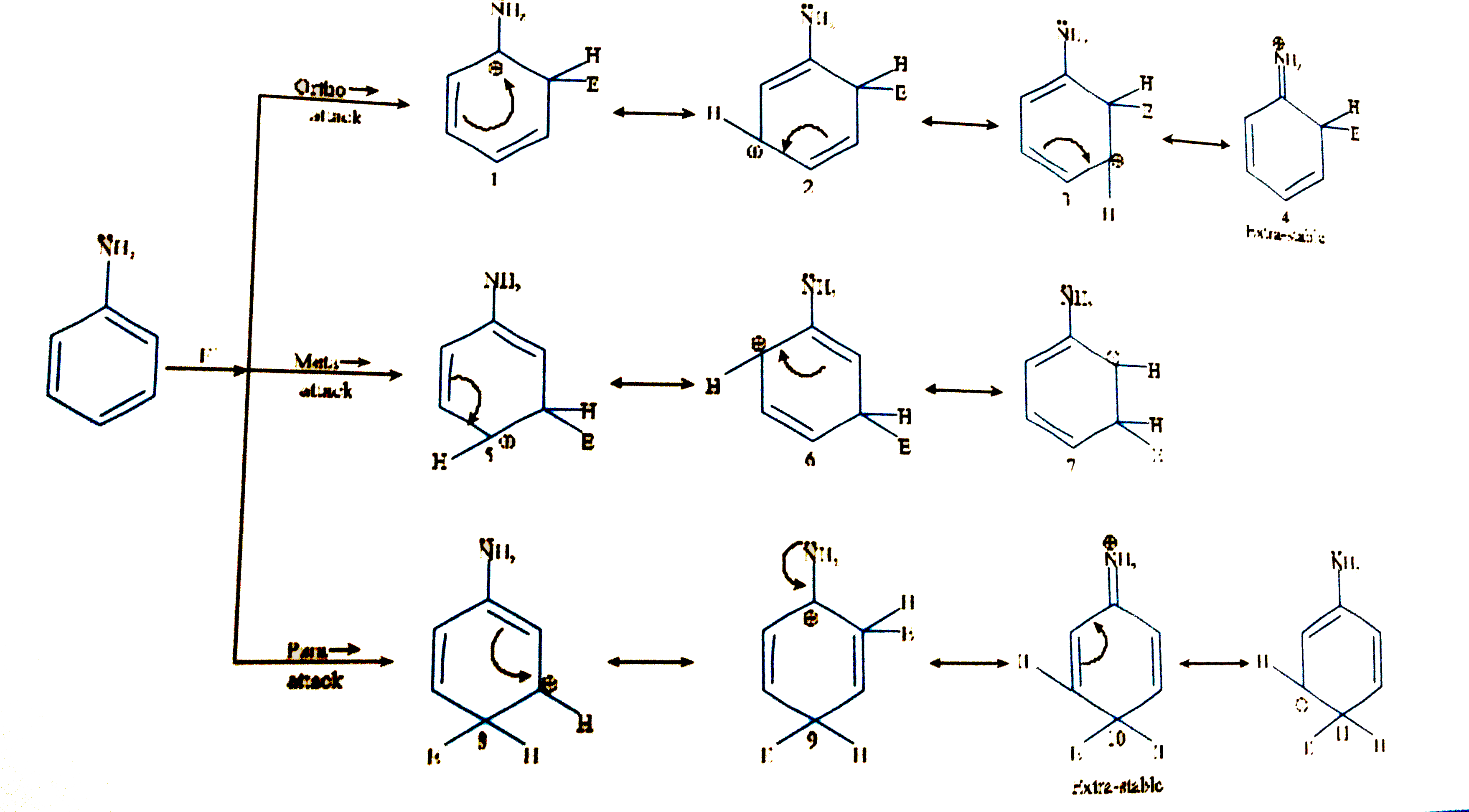
In case of meta-attack on aniline (where −NH2 group is o−, p− directing) and in case of ortho-attack on nitrobenzene, resonating structures are possible. Which of them are more stable ?
_E01_264_Q01.png)
The correct Answer is:(I)
Topper's Solved these Questions
Similar Questions
Explain the following :
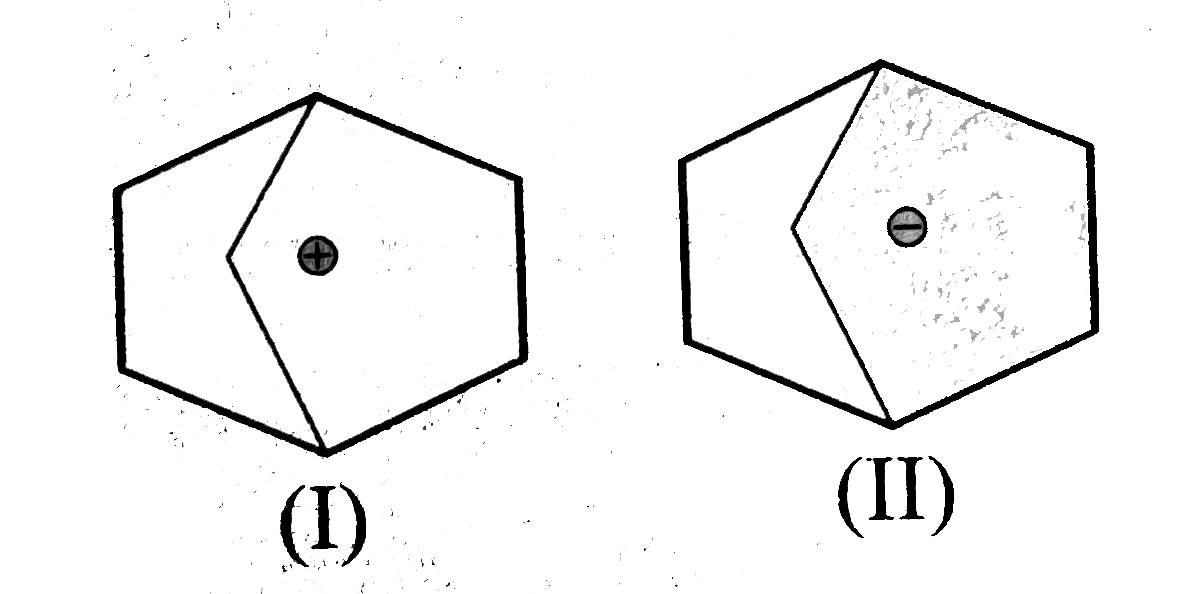
(a) Why MeNH⊕CH2 (I) is more stable more than MeCH2⊕CH2 (II) when both are 1∘ carbocations ?
(b) Which of the following intermediate is unstable ?
( c) Why allylic free radical (CH2−CH=CH2)(I) is more stable than propy1 free radical (.CH2−CH2−CH3)(II), when both are 1∘ free radicals ?
(d) Arrange the following in the decreasing order of their stabilities :

( e) Which of the following resonance structures of p−nitro phenoxide is not feasible ?
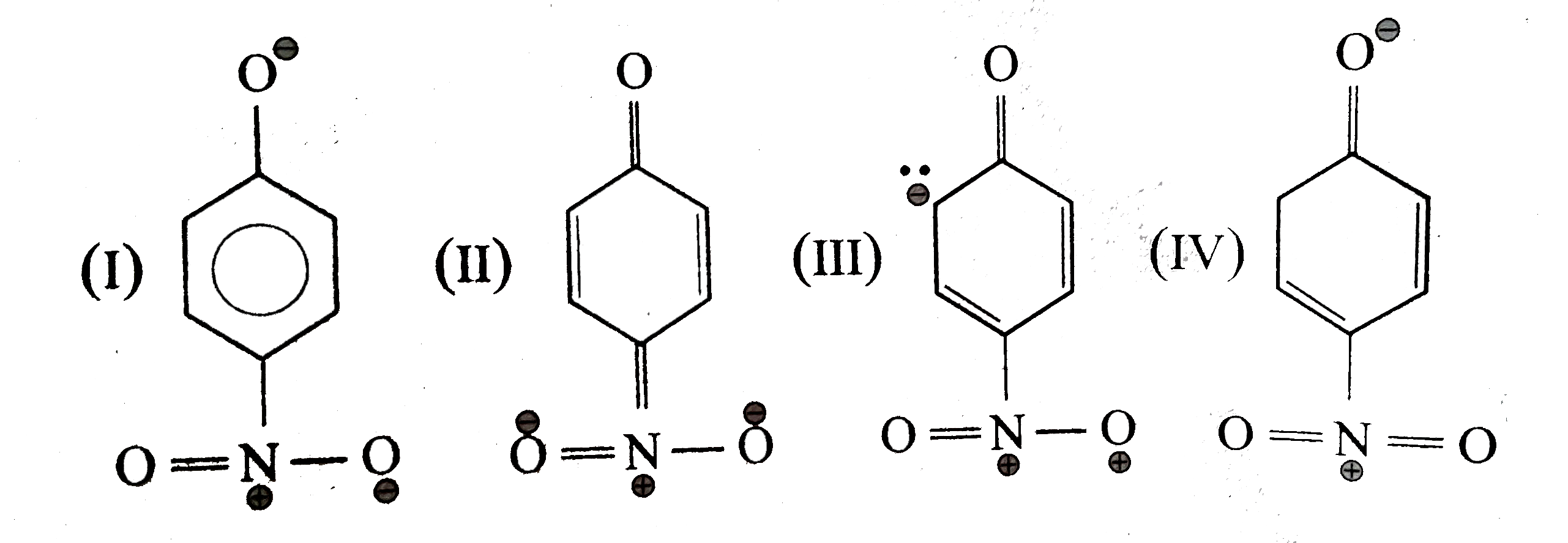 .
.
(f) Which of the more stable carbocation ?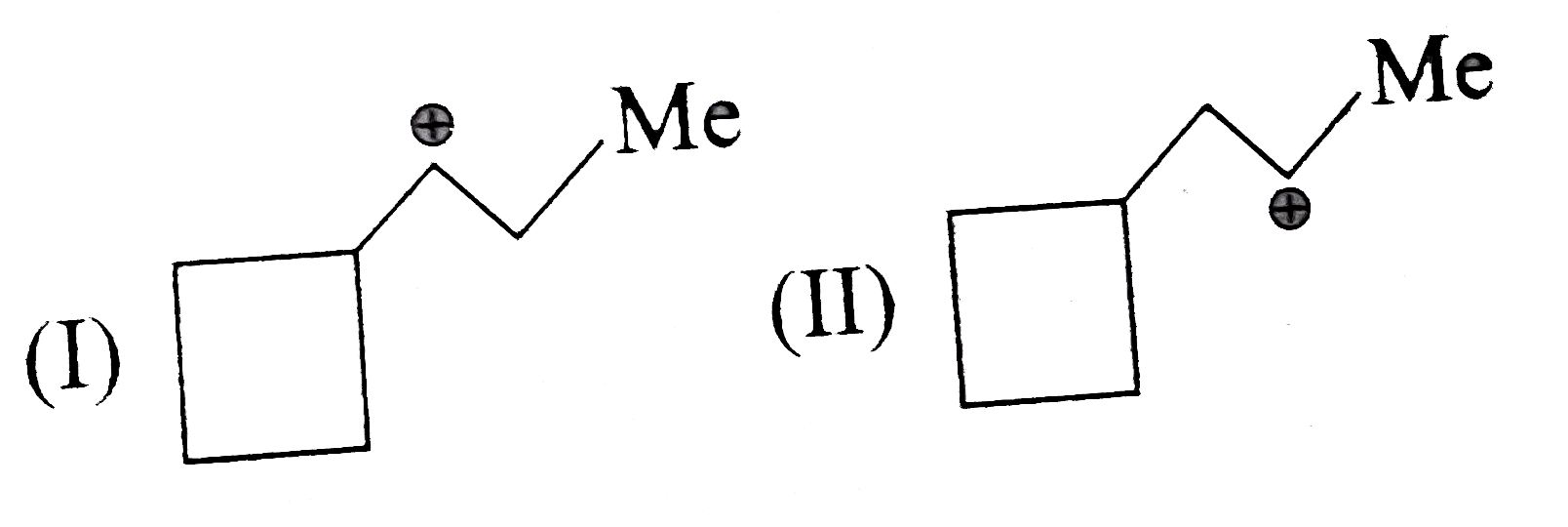 .
.
View Solution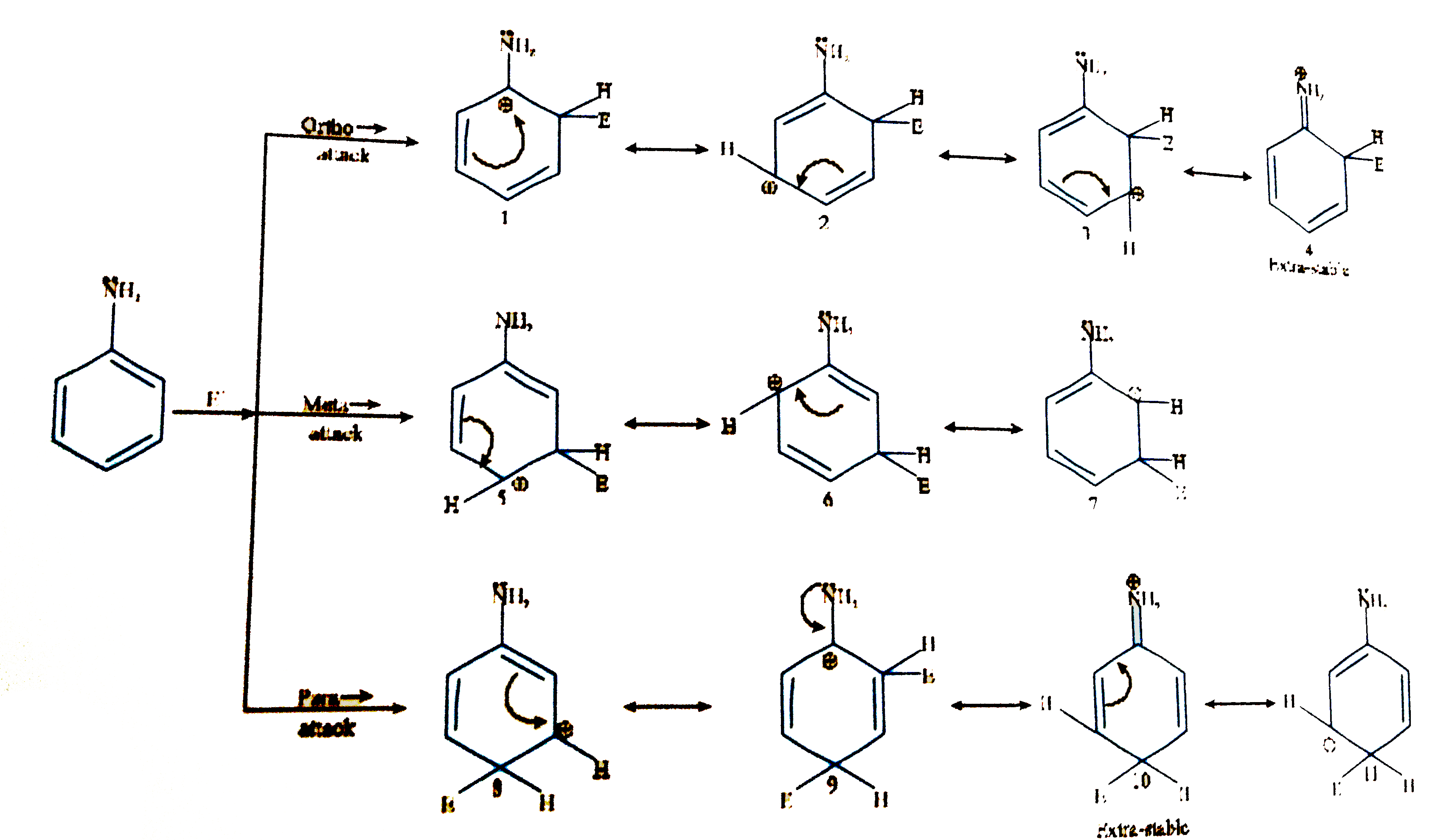
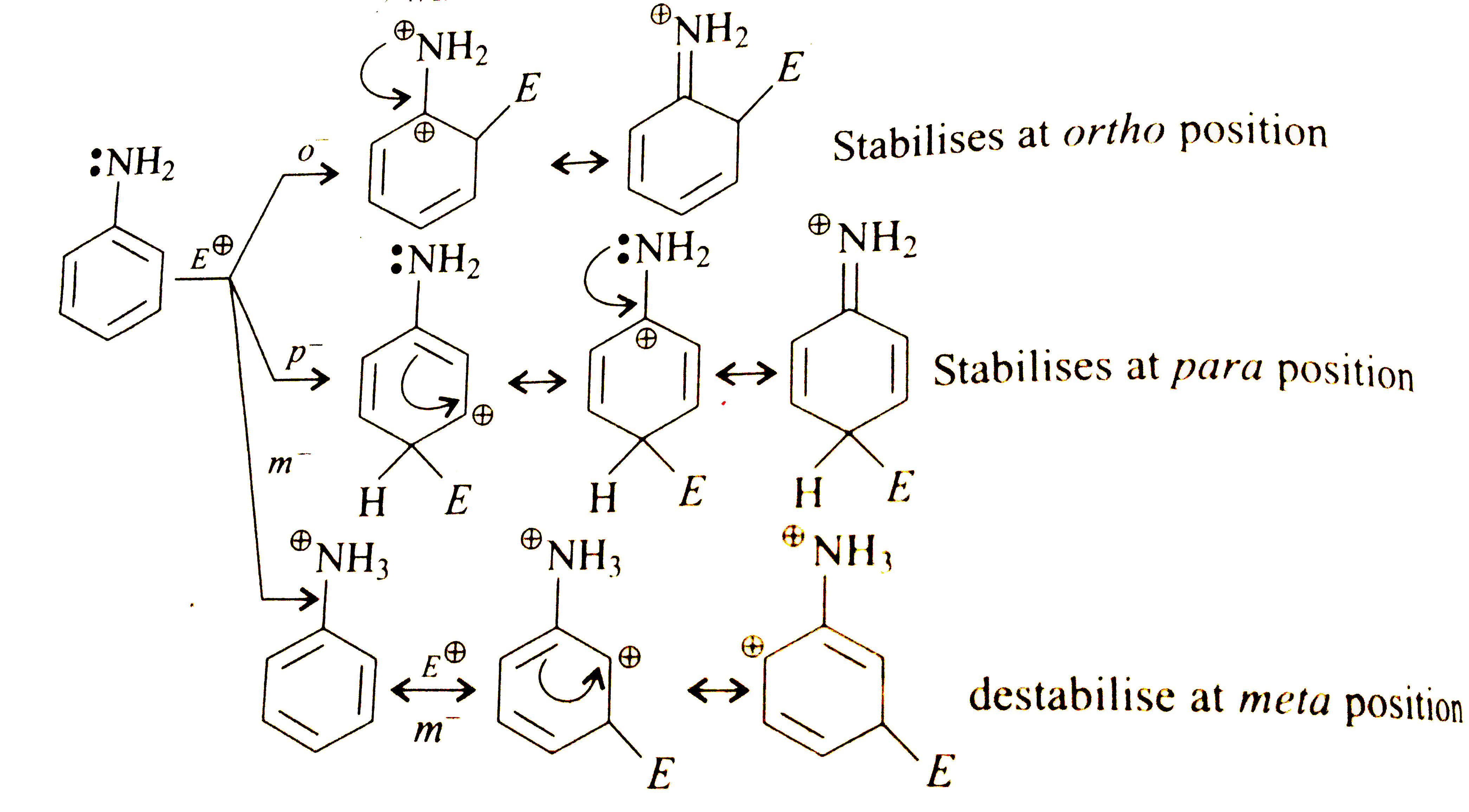
Directing nature of substituted aromatic compound is decided by stability of σ-complex or areniumion. If σ-complex is stabilised at O-and P-position by attacks of electrophile then the group is O-and P-directing, but if σ-complex si stabilisd at m-position the group will be meta directing on the basis of above explanation. find out correct answers of following questions.
Q. Which of the followin is -O- and p-directing
View Solution
Knowledge Check
In which of the following, resonance of −NH2 group is possible ?
A1-AminobutaneBEthylamineCBenzylamineDp-ToluidineAmino group, −NH2 is ortho, para-directing group in case of aromatic electrophilic substitution but nitration of aniline produce a good amount of m-nitroaniline. This is because
A−NH2 gets converted into - NH−NO+2 which is m-directingBNH2 gets converted into +NH3 which is m-directingC−NH2 gets converted into −NH+SO−4 which is m-directingDortho, para activity of −NH2 group is completely destroyed during nitrationDirecting nature of substuted aromatic compound is decided by stability of σ-complex is stablise at o- and p- position by attack of electrophile then group is o- and p- directing but if σ-complex is stabilise at m- position then gruop will be meta directing.
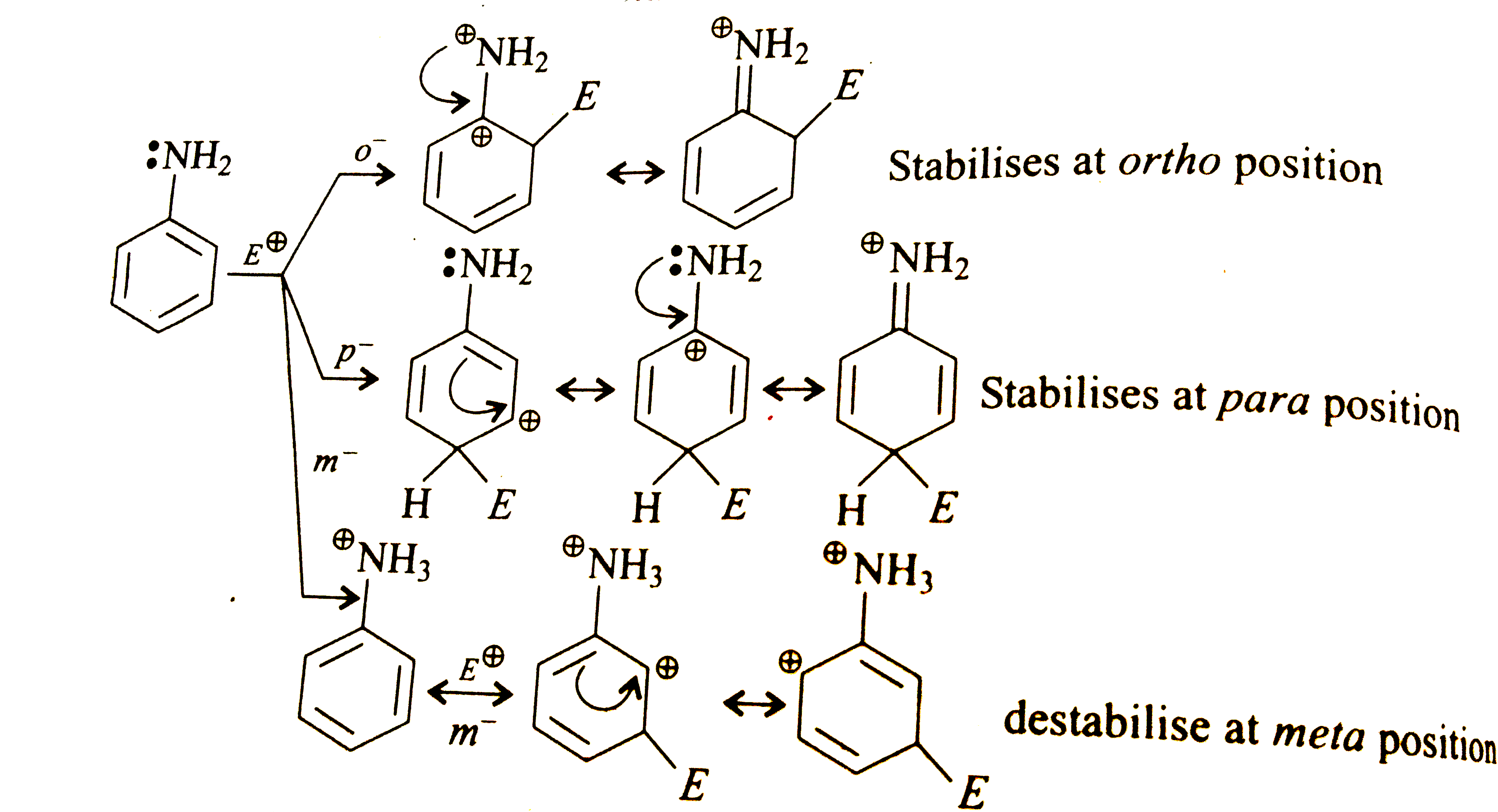
On the basis of above explanation find out correct answer of following questions.
Which of the following is m-directing ?A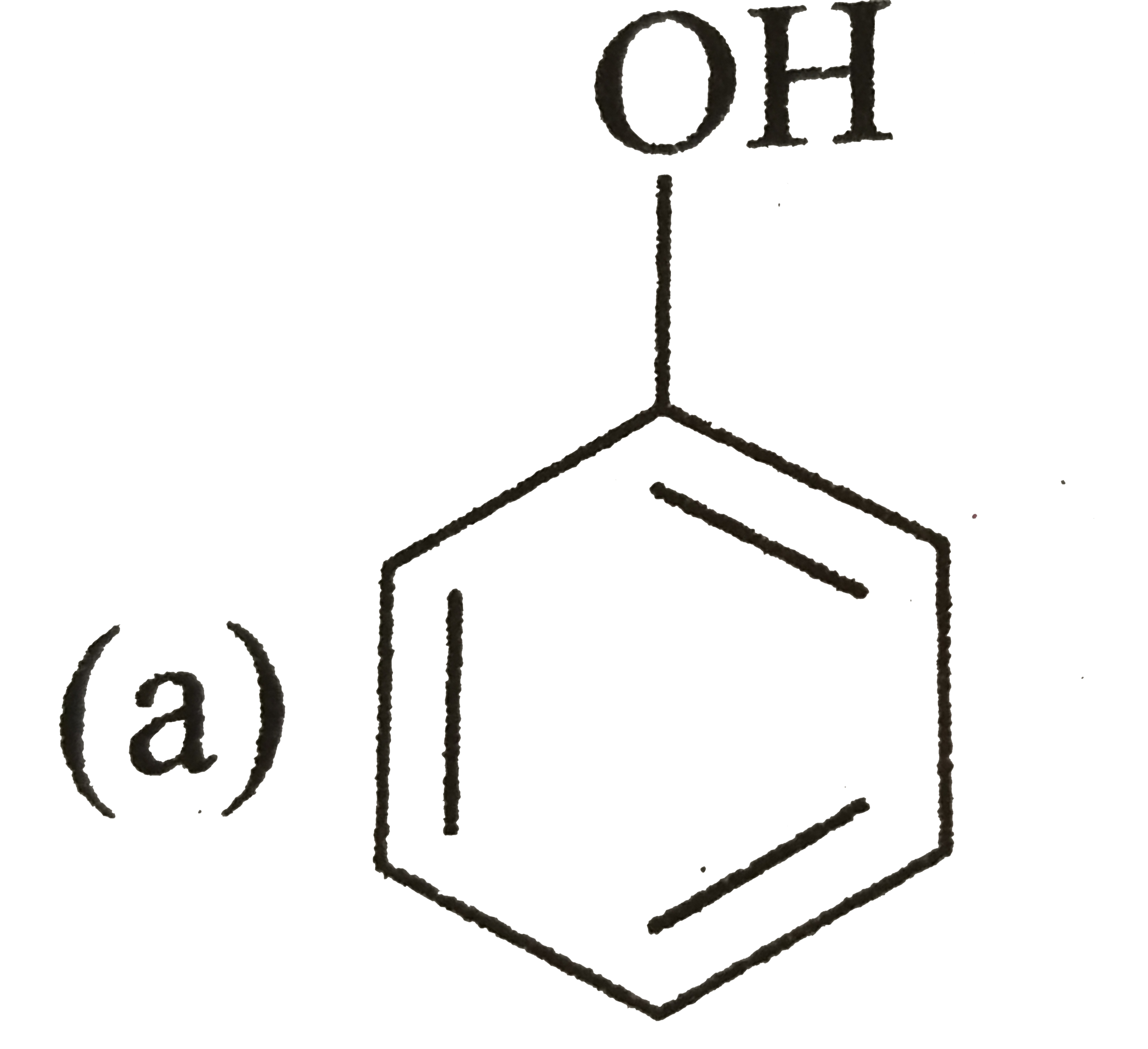 B
B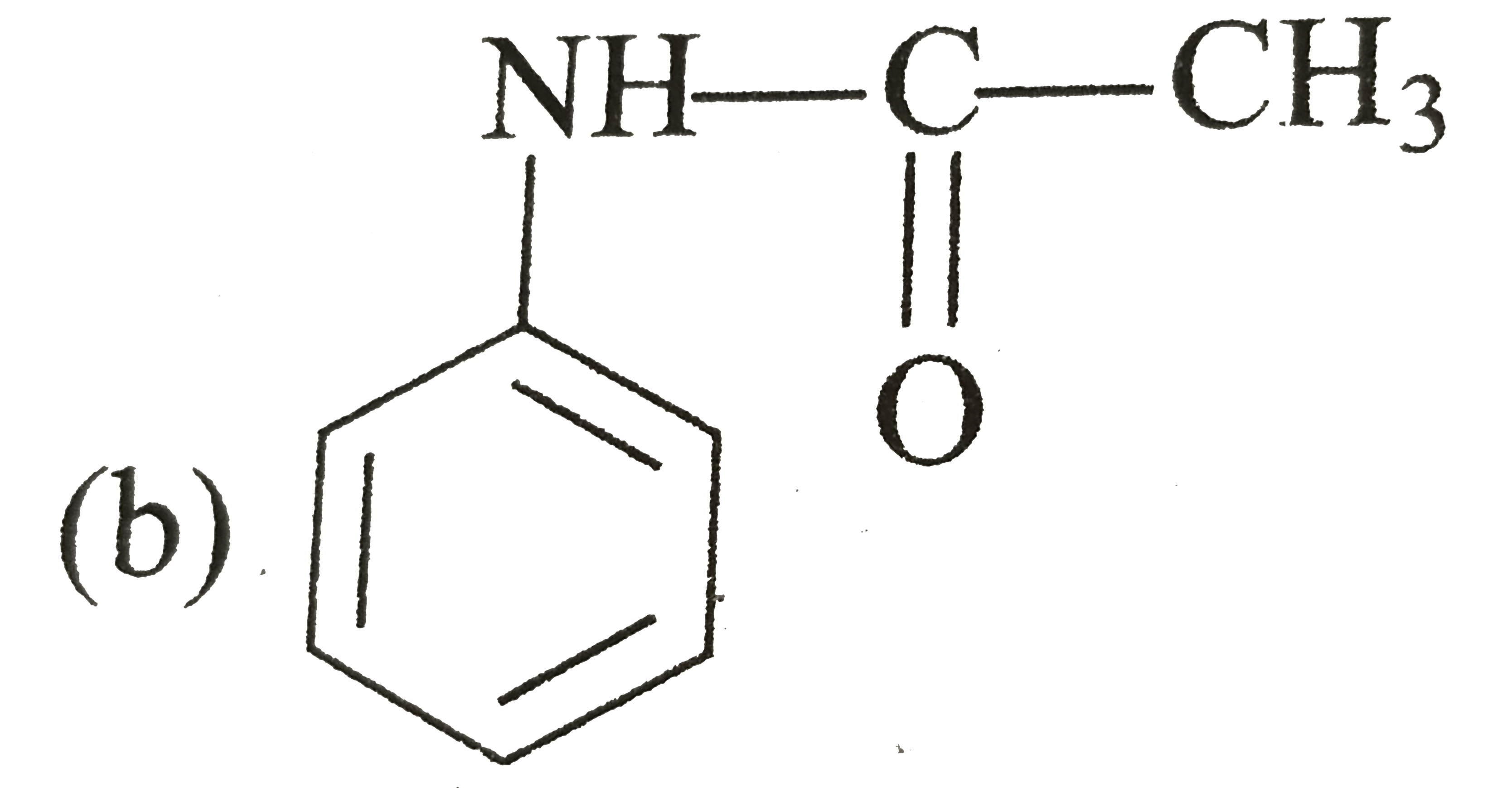 C
C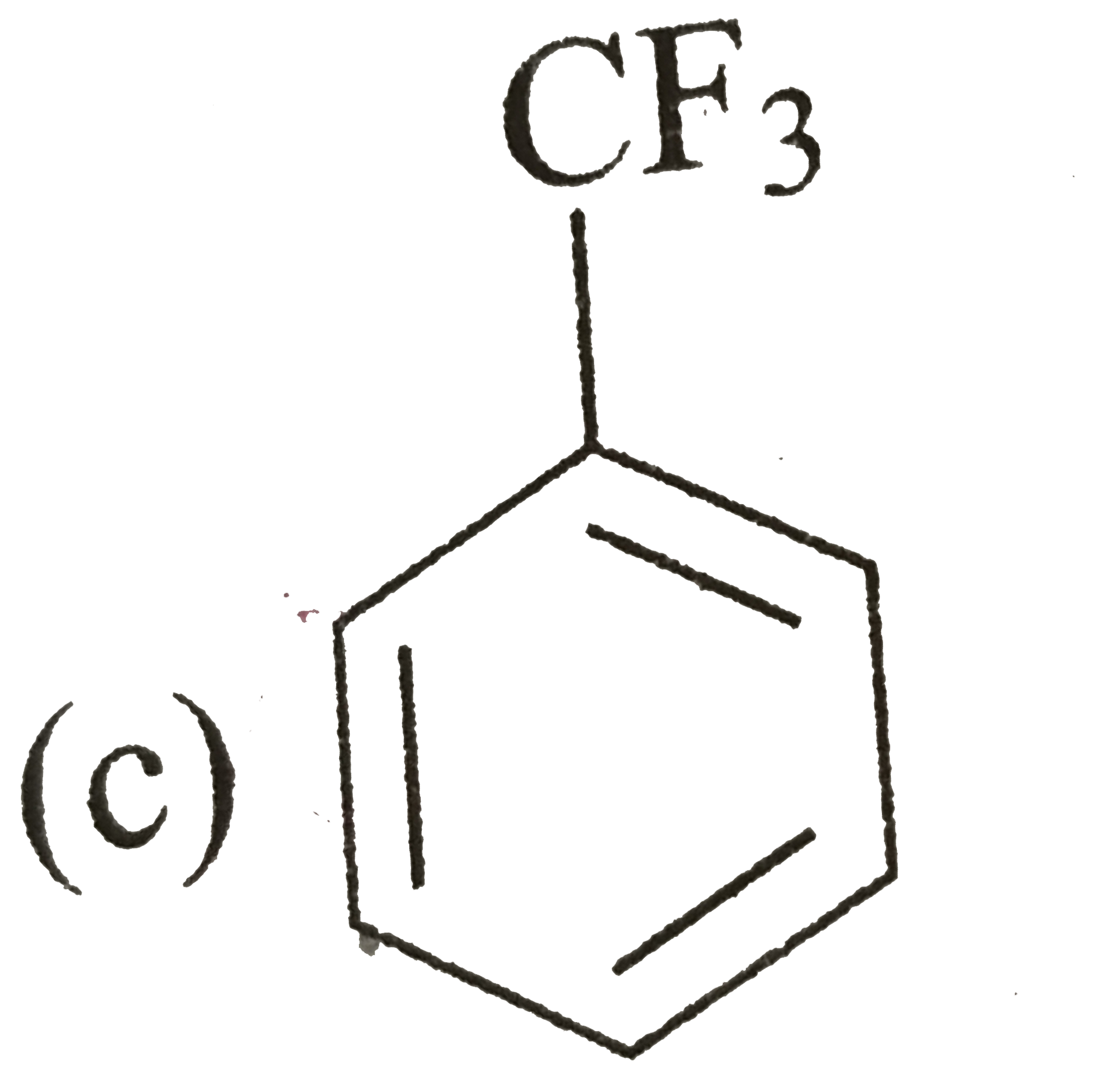 D
D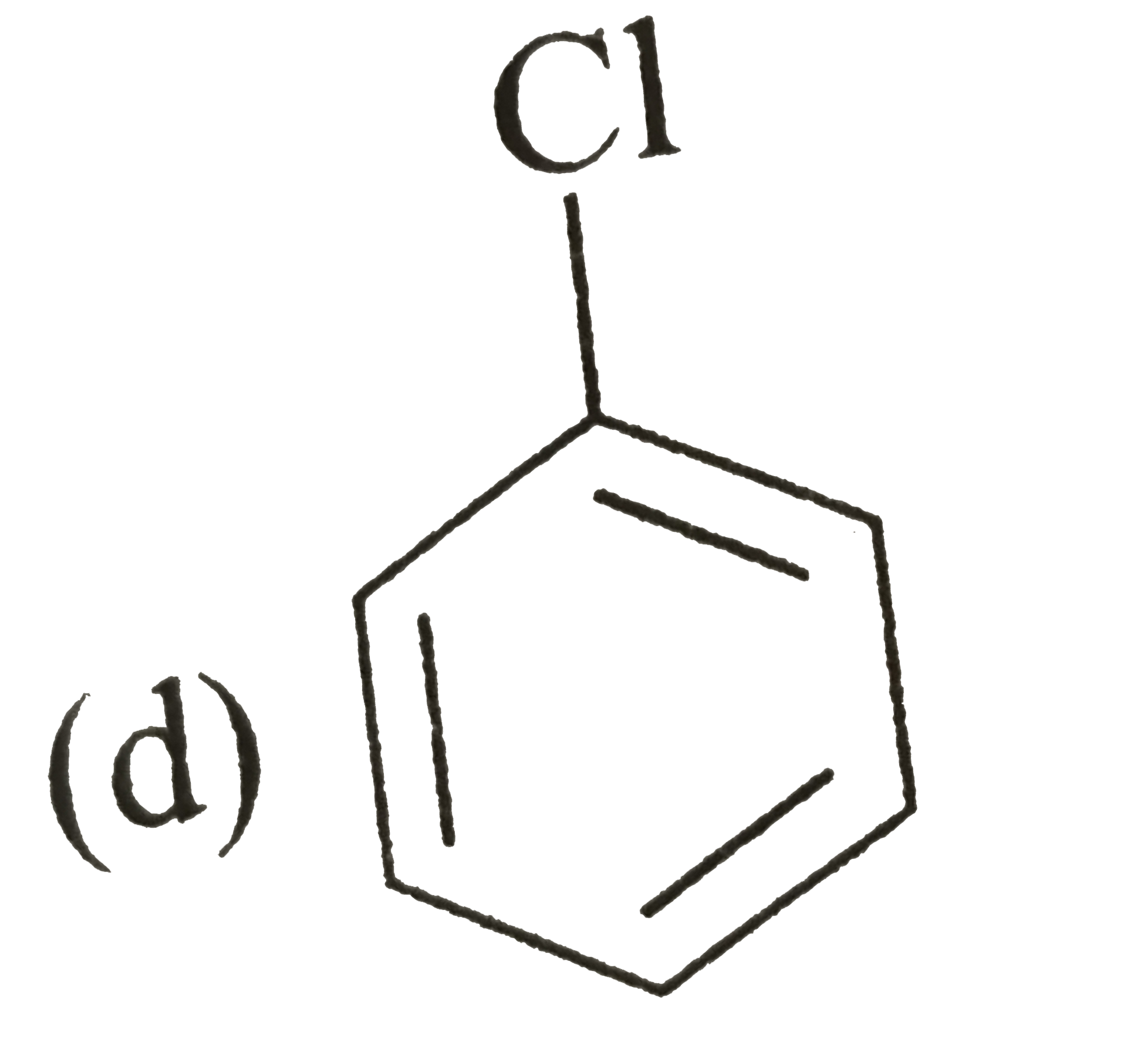
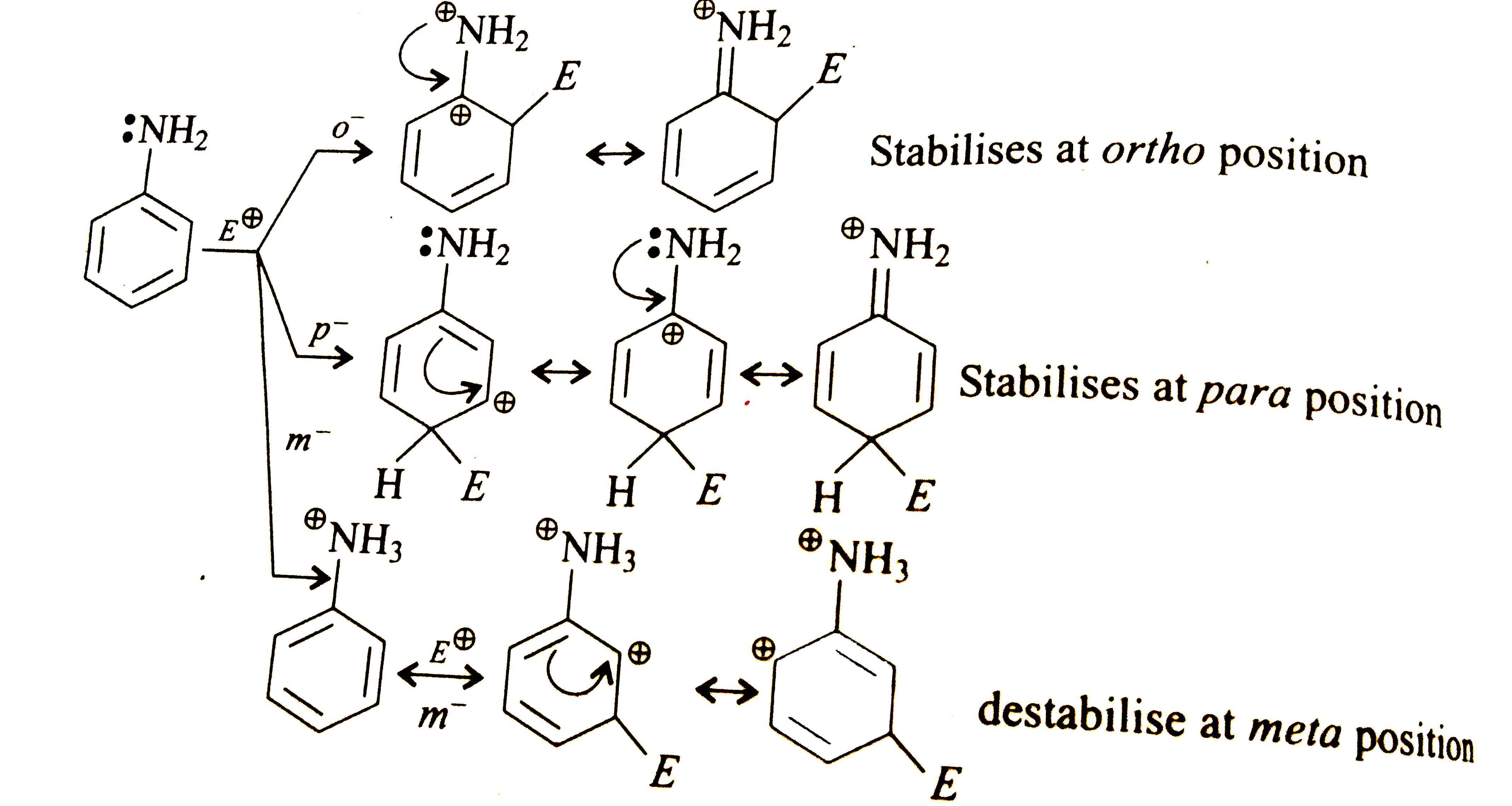
Directing nature of substituted aromatic compound is decided by stability of σ-complex or areniumion. If σ-complex is stabilised at O-and P-position by attacks of electrophile then the group is O-and P-directing, but if σ-complex si stabilisd at m-position the group will be meta directing on the basis of above explanation. find out correct answers of following questions.
Q. Which of the following is not O- and P-directing
View SolutionDirecting nature of substuted aromatic compound is decided by stability of σ-complex is stablise at o- and p- position by attack of electrophile then group is o- and p- directing but if σ-complex is stabilise at m- position then gruop will be meta directing.
On the basis of above explanation find out correct answer of following questions.
Which of the following is not o- and p- directing ?
View SolutionDirecting nature of substuted aromatic compound is decided by stability of σ-complex is stablise at o- and p- position by attack of electrophile then group is o- and p- directing but if σ-complex is stabilise at m- position then gruop will be meta directing.
On the basis of above explanation find out correct answer of following questions.
Which of the following is o- and p-directing
View SolutionIn the electrophilic substituion of benzene ring, the second substituent is directed by the group already present. Electron releasing groups (+I and +M) are ortho-para-directing and activating, whreas the electron withdrawing groups (-I and -M) are meta-directing and deactivating.
Halogens are placed under the category of +T (Tautomeric) groups because they have -Ibdyctuve abd +Mesomeric effect. These groups are deactivating but ortho-para-directing.
In the introduction of third group to the benzene ring, the product of minimum steric gindrance is formed.
Which of the following substituted benzene derivatives would furmish three isomers when one more substituent is introduced ?
View SolutionConsider the following statements concerning orthq and para hydrogen.
1. Ortko is less stable man para.
2. Vapour pressure ofortho is more than of para,
3. Magnetic moment ofpara is zero while in case of ortho it is twice that of a proton.
4. Conductivity of para is less than that ofortho.
Which of these statements are correct ?
View Solution

