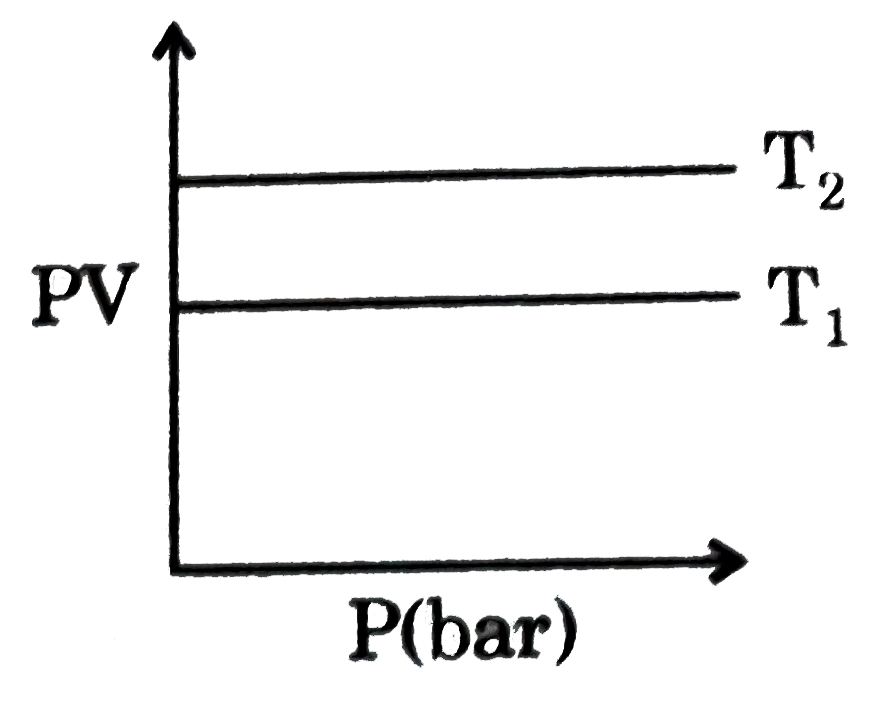
For a reaction, A⇔P, the plots of [A[ and [P] with time at temperature T1andT2 are given ahead:
![For a reaction, A hArr P, the plots of [A[ and [P] with time at temperature T(1) and T2 are given ahead: . If T(1) gt T(1), the correct statement(s) is (are): (Assume DeltaH^(@) and DeltaS^(@) are independent of temperature and ratio of ln K at T(1) to ln K at T(2) as greater than (T(2))/(T(1)). Here, H,S,G and K are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively.](https://d10lpgp6xz60nq.cloudfront.net/physics_images/GRB_PHY_CHM_P1_C04_E01_259_Q01.png) .
.
If T1>T1, the correct statement(s) is (are):
(Assume ΔH∘ and ΔS∘ are independent of temperature and ratio of ln K at T1 to ln K at T2 as greater than T2T1. Here, H,S,G and K are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively.
A
ΔH∘<0,ΔS∘<0
B
ΔG∘<0,ΔH∘>0
C
ΔG∘<0,ΔS∘<0
D
ΔG∘<0,ΔS∘>0
Text Solution
The correct Answer is:A, C
Given that:
ln K1ln K2>T2T1 (Here T2>T1)
T1 ln K1>T2lnK2
RT1lnK1>RT2lnK2 (It shows that ΔG∘<0)
(−ΔH∘−T1ΔS∘)>−(ΔG∘−T2ΔS∘)
−ΔH∘+T1ΔS∘>−ΔH∘+T2ΔS∘
Above expression will be correct ony when (ΔH∘<0)
T1ΔS∘>T2ΔS∘
Given that T2>T1
∴ΔS∘<0
|
Similar Questions
Knowledge Check
A black body radiates heat at temperatures T1 and T2(T2>T1 the frequency corresponding to maxium energy is
Amore atT1Bmore at T2Cequal for T1 and T2Dindependent of T1 and T2A black body radiates heat at temperatures T1 and T2(T2>T1 the frequency corresponding to maxium energy is
Amore atT1Bmore at T2Cequal for T1 and T2Dindependent of T1 and T2Shown below are the black body radiation curves at temperature T1 and T2(T2>T1). Which of the following plots is correct?
A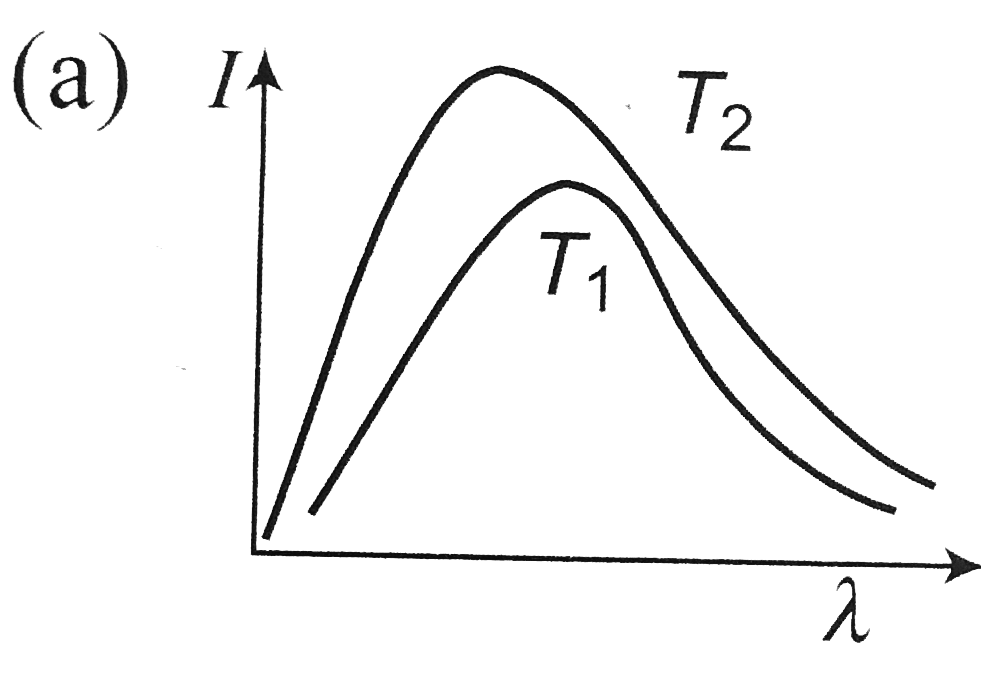 B
B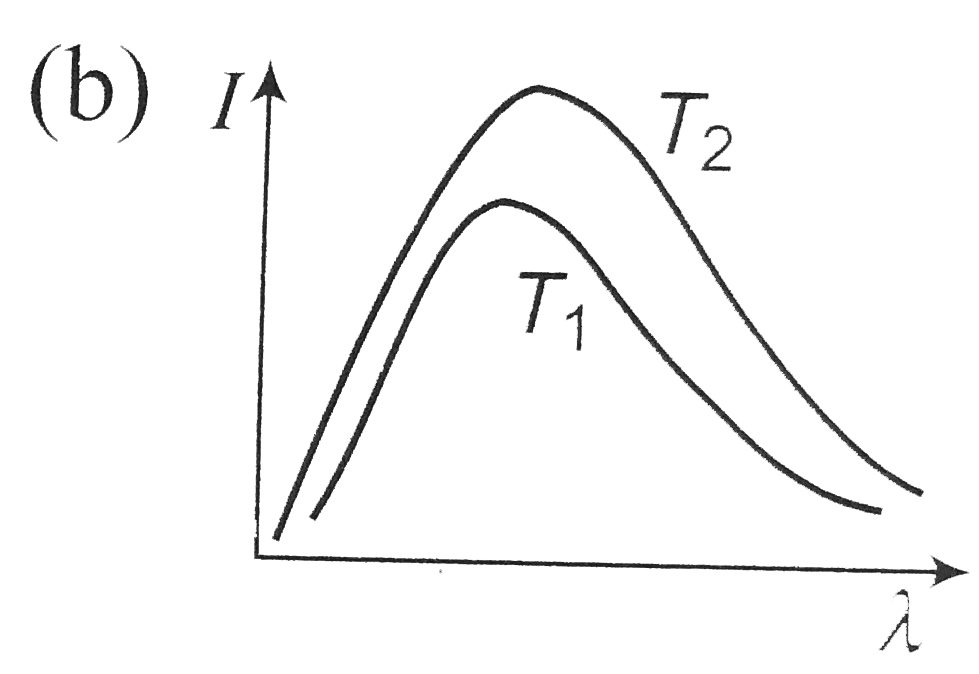 C
C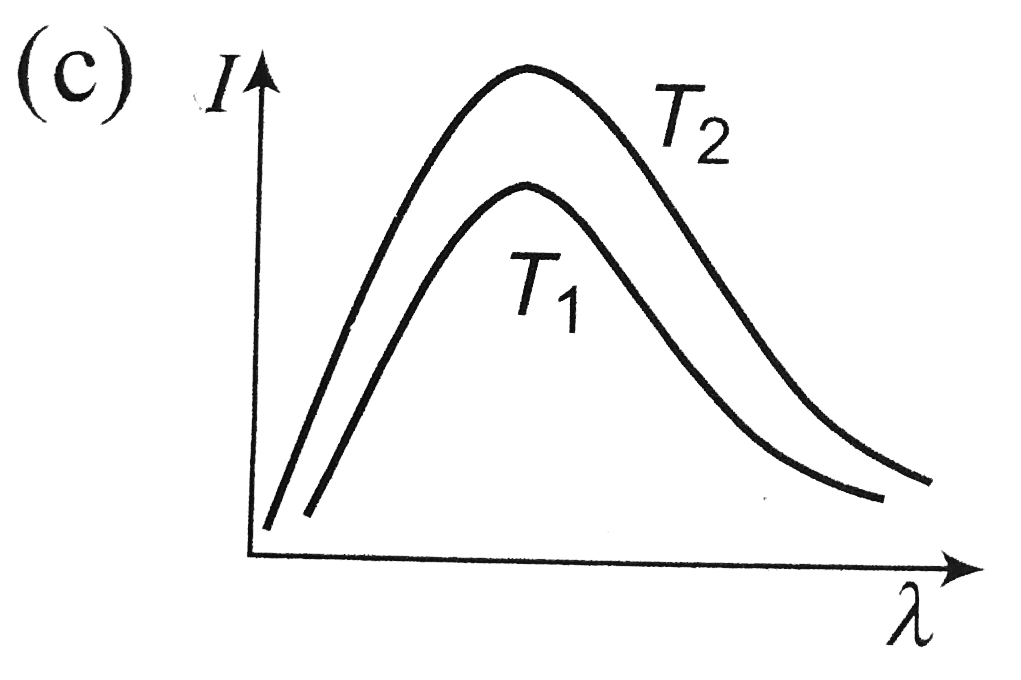 D
D
Select the incorrect statements about the plots of ln K vs 1/T
View Solutionχ1 and χ2 are susceptinilities of diamagnetic substance at temperatures T1K and T2K respectively. Then
View SolutionThe amount of heat energy required to raise the temperature of 1 g of Helium at NTP, from T1 K to T2 K is :
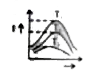
View SolutionThe plots of intensity v/s wavelength for three at temperatures T1,T2 & T3 respectively. Their temperatures are such that (Decreasing order)
View SolutionFor a given exothermic reaction , Kp and k'p are the equilibrium constants at temperatures T1 and T2 respectively. Assuming that heat of reaction is constant in temperature range reaction is constant in temperature range between T1andT2 , it is readily observed that
View Solution