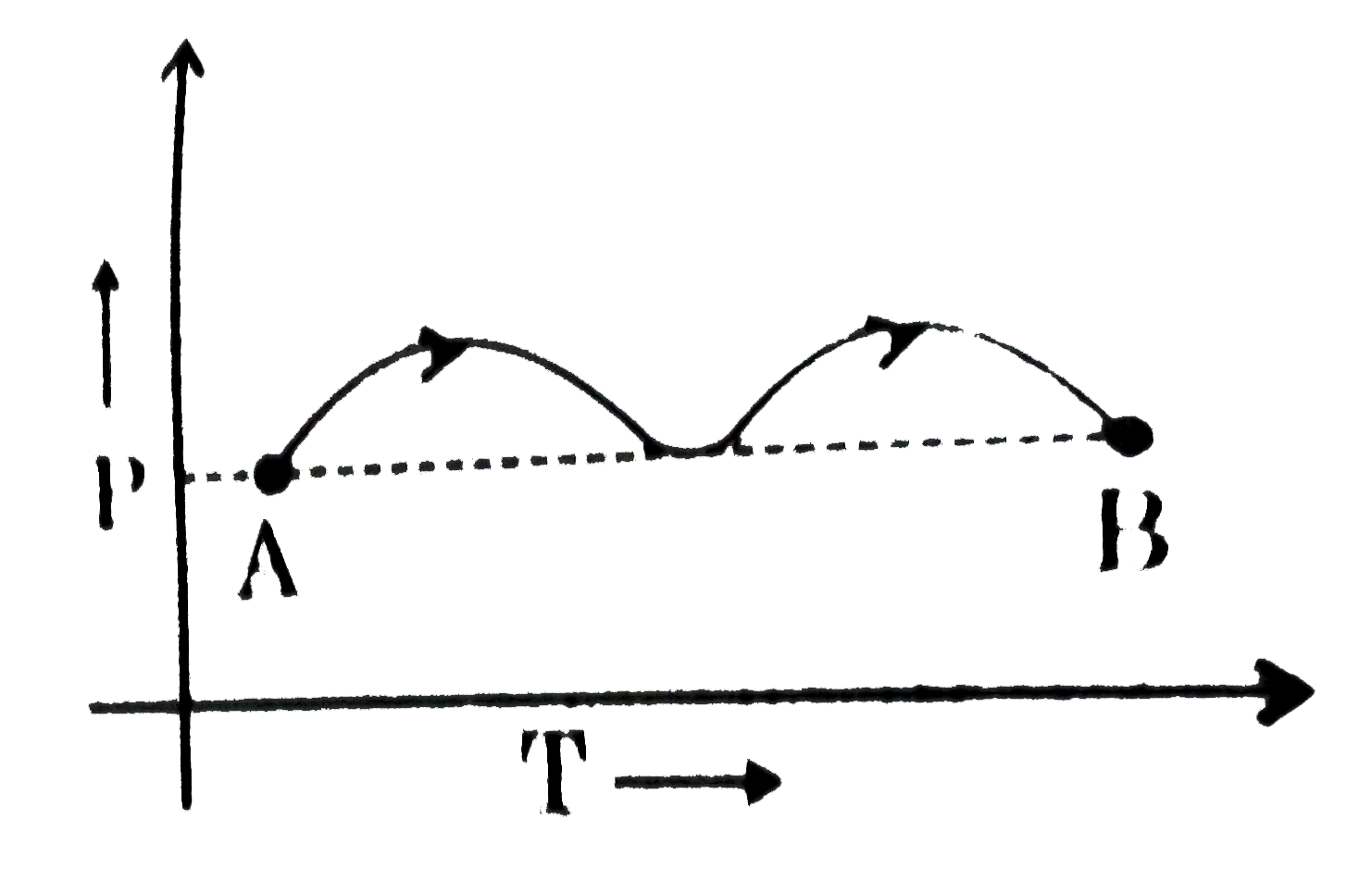
The P-T graph, as given below, was observed for a process on an ideal gas. Which of the statement is true ?
A
w=+ve,ΔH=+ve
B
w=−ve,ΔH=−ve
C
w=−ve,ΔH=+ve
D
w=+ve,ΔH=−ve
Video Solution
Text Solution
The correct Answer is:C
From graph we know that VB>VA. So expansion has taken place so w will be with −ve sign and ΔH will be +ve as both ΔE and Δ(PV) have increased.
|
Similar Questions
Knowledge Check
The P-T graph, as given below, was observed for a process on an ideal gas. Which of the statement is true ?
 Aw=+ve,ΔH=+veBw=−ve,ΔH=−veCw=−ve,ΔH=+veDw=+ve,ΔH=−ve
Aw=+ve,ΔH=+veBw=−ve,ΔH=−veCw=−ve,ΔH=+veDw=+ve,ΔH=−veThe graph given below shows the P-V plot for a process on an ideal gas. Select the correct statement.
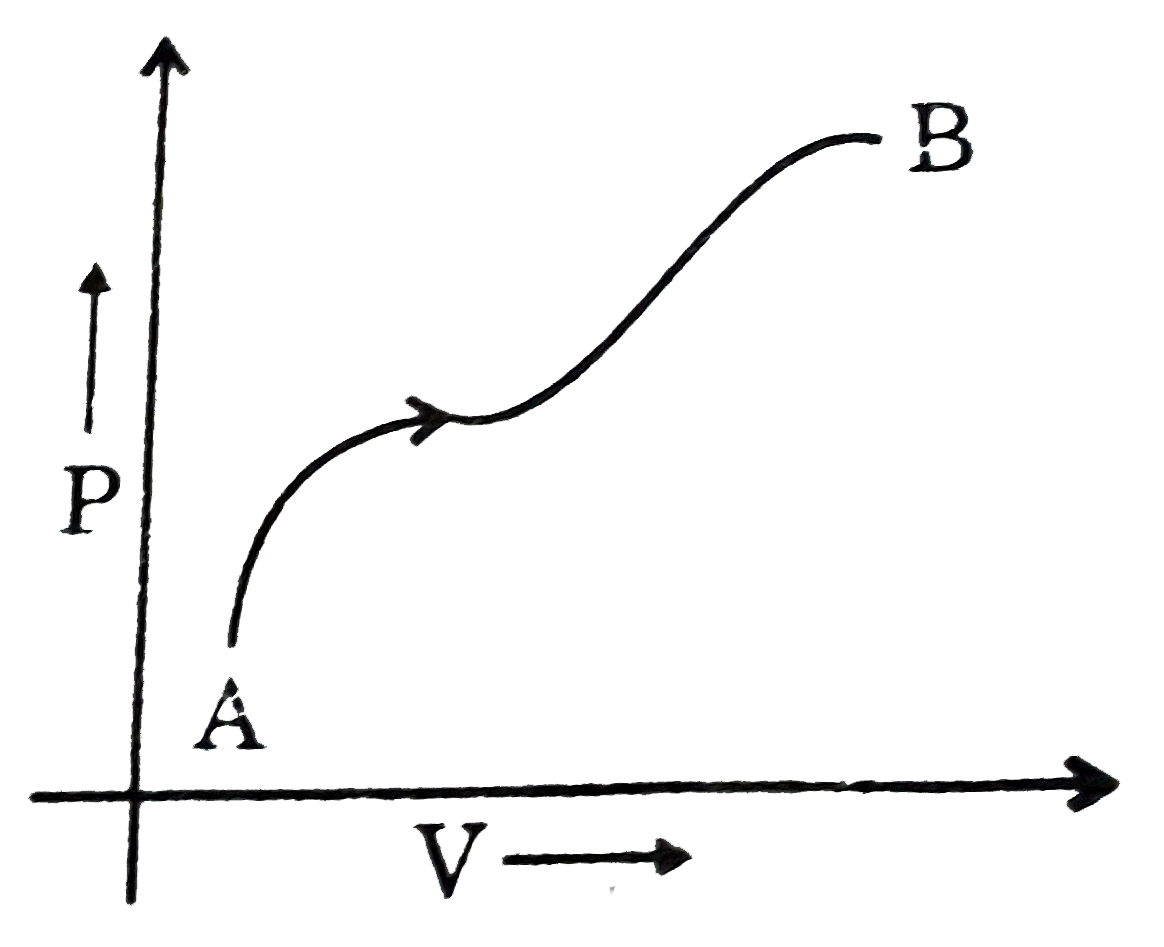 AEnthalpy content of the gas is constantly increasing and the process is carried out slowlyBEnthalpy content of the gas first increases then decreases and the process is quasistaticlyCEnthalpy content of the gas is constant and the process taken infinite amount of time for completionDEnthalpy content first decreases then increases and the process is reversible
AEnthalpy content of the gas is constantly increasing and the process is carried out slowlyBEnthalpy content of the gas first increases then decreases and the process is quasistaticlyCEnthalpy content of the gas is constant and the process taken infinite amount of time for completionDEnthalpy content first decreases then increases and the process is reversibleOne mole of an ideal gas is taken through a cyclic process as shown in the V-T diagram. Which of the following statements is true ?
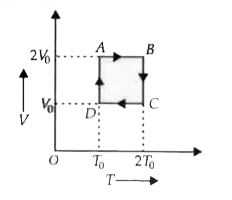 AThe magnitude of work done by the gas is RT0ln2.BWork done by gas is V0T0CNet work done by the gas is zero.DWork done by the gas is 2RT0ln2.
AThe magnitude of work done by the gas is RT0ln2.BWork done by gas is V0T0CNet work done by the gas is zero.DWork done by the gas is 2RT0ln2.
One mole of a monatomic ideal gas undergoes a cyclic process as shown in the figure (where V is the volume and T is the temperature). Which of the statements below is (are) true ?
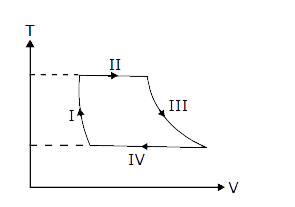
View SolutionThe distance-time graph for the motion of two vehicles A and B is given below. Which one of the following statements is true ?
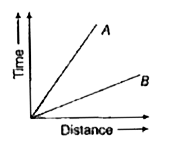
View SolutionThe P−T graph for the given mass of an ideal gas is shown in figure. Then the volume
_E01_022_Q01.png)
View SolutionThe distance - time graph for the motion of two vehicles . A and B is given below. Which one of the following statements is true ?
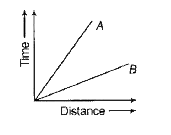
View SolutionOn the P−T graph of an ideal gas, choose the correct option(s):
View Solution

