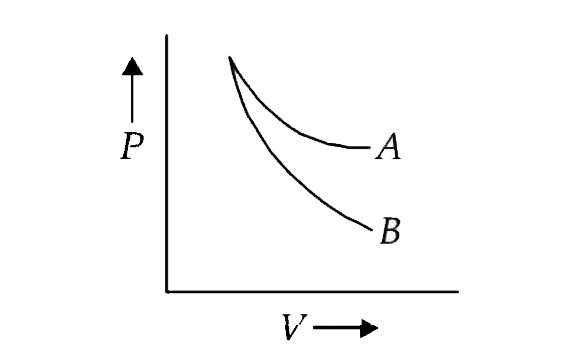
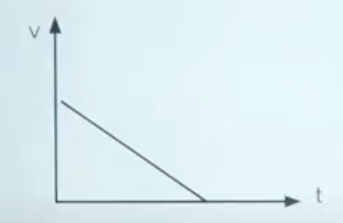
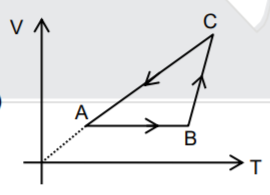
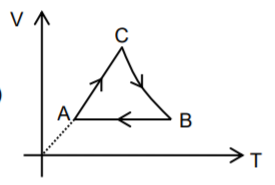
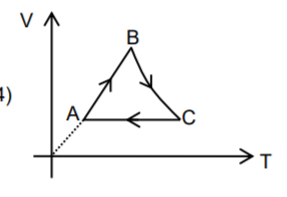
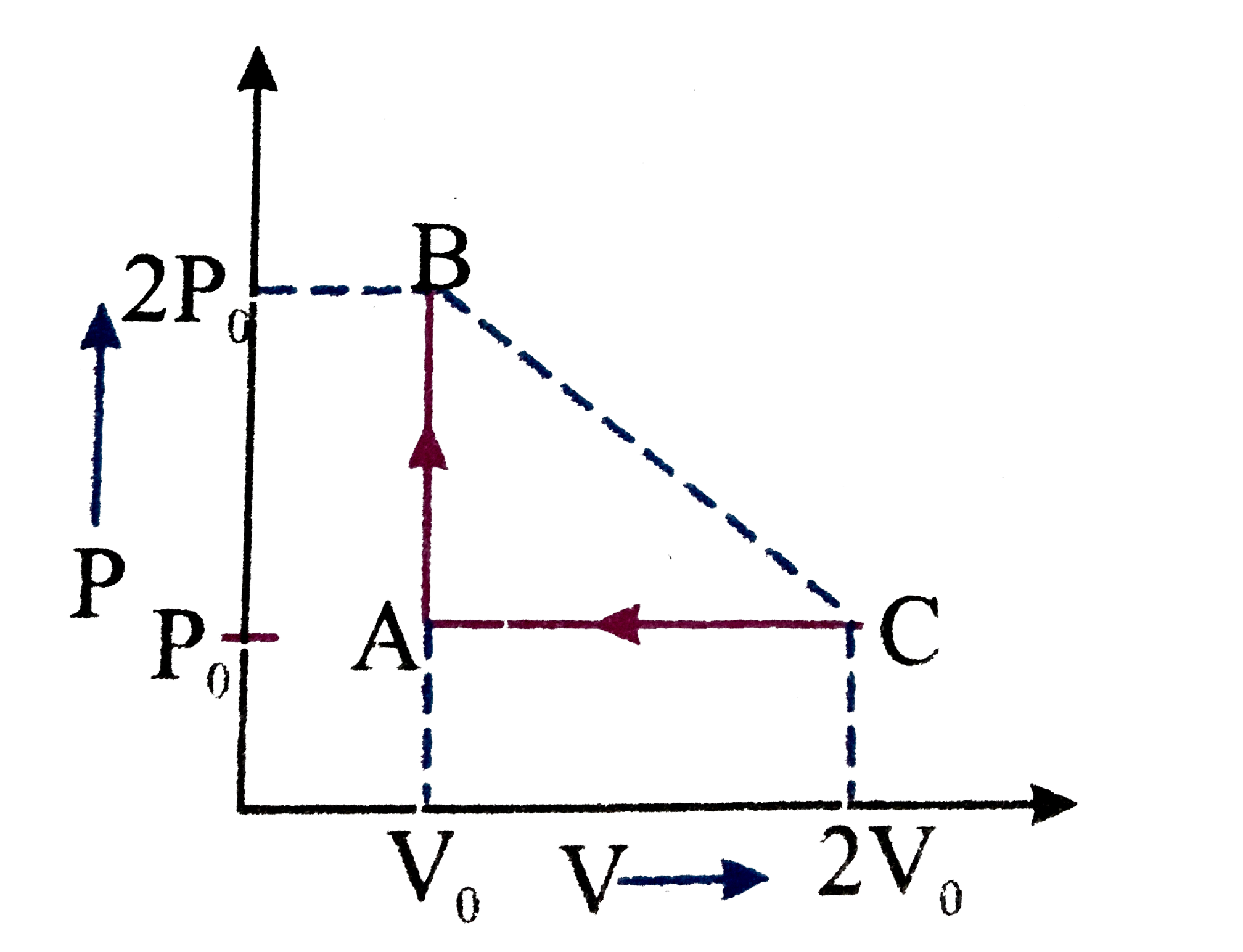
For the given P-V graph for an ideal gas, chose the correct V-T graph. Process BC is adiabatic. 
A
B
C
D
Video Solution
Text Solution
The correct Answer is:A
NA
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
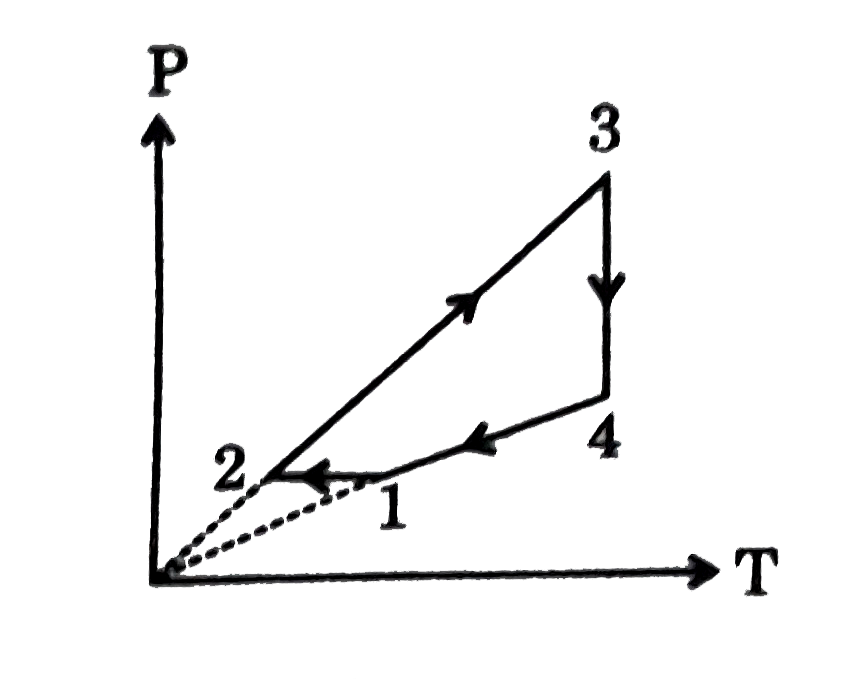
On the P−T graph of an ideal gas, choose the correct option(s):
AAdiabatic process will be a straight line.BIsochoric process will be straight line passing through the origin.Cadiabatic curve will have a positive slope.Dthe slope of adiabatic curve is proportional T.Consider the following (P-T) graph for a fixed mass of gas: Correct P-V graph as:
 A
A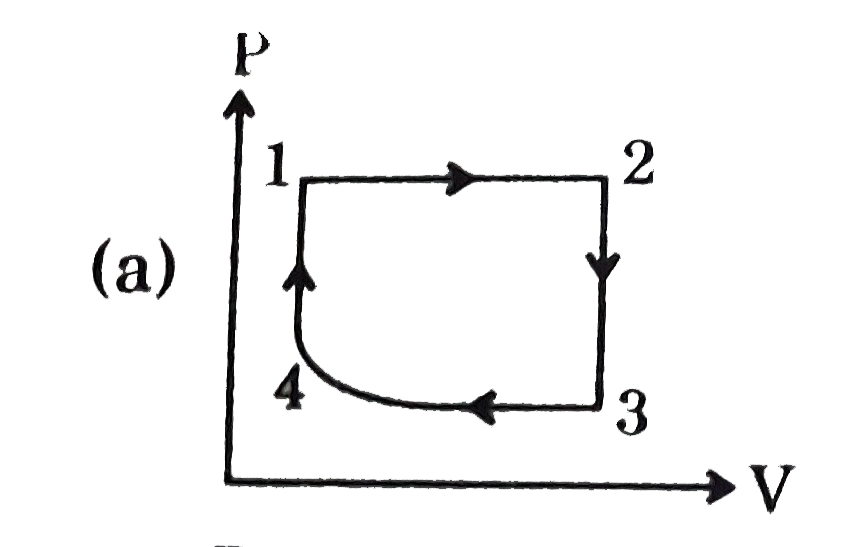 B
B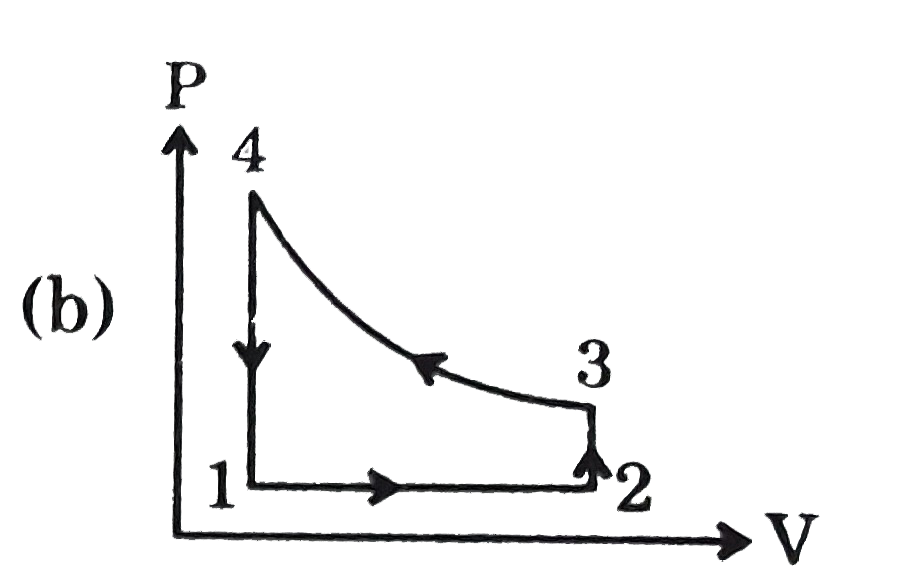 C
C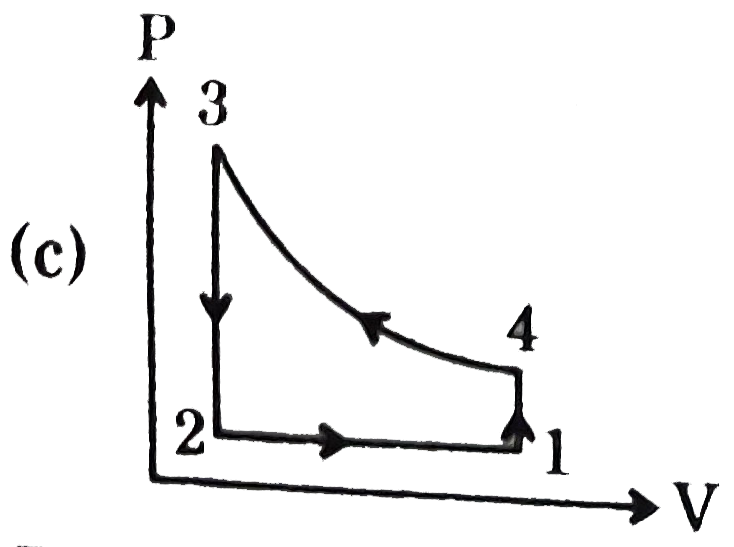 D
D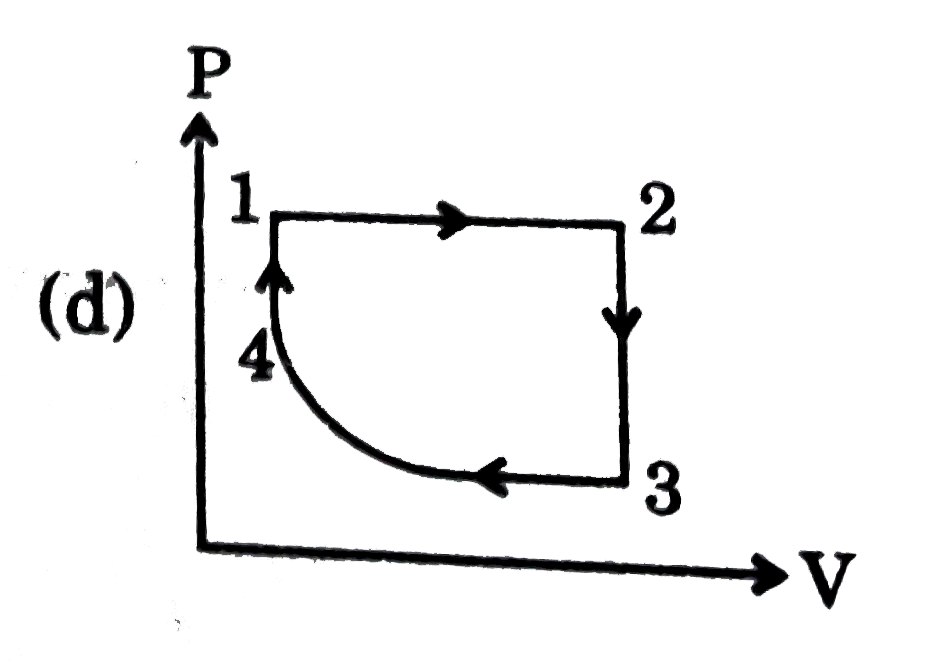
Straight line on (p - T) graph for an ideal gas represents isochoric process.
If p∝T,V=constant.AStatement-1 is true, statement-2 is true, statement-2 is a correct explanation for statement-1BStatement-1 is true, statement-2 is true, statement-2 is not a correct explanation for statement-1Cstatement-1 is true, statement-2 is falseDstatement-1 is false, statement-2 is true
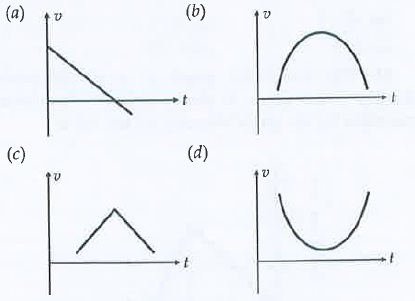
A particleis thrown above, the correct v-t graph will be
View SolutionThe P - V graph of an ideal gas cycle is shown here as below. The adiabatic process is described by

View SolutionStraight line on (p - T) graph for an ideal gas represents isochoric process.
If p∝T,V=constant.
View SolutionThe figure shows P-V graph of an ideal on molegas undergone to cyclic process ABCD then the process B→C is
View SolutionThe slope of P-V graph for isochoric process is
View Solution