Which of the following option the first compound has less entropy than second:
A
(i) aqueous solution of 1M ofMgCI2(ii) aqueous solution of 1M ofNaCI
B
(1)Br2 liquid at 25∘(ii)Br2 liquid at 20∘C
C
(i)HgO solid (ii)HgS solid
D
(i)Br2 liquid (ii)I2 solid
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
In which of the following pair first compound has more resonance energy than second ?
A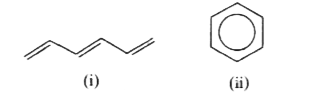 B
B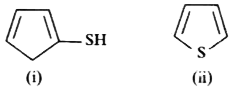 C
C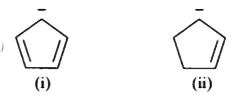 D
D
In which of the following pair first compound has more resonance energy than second ?
A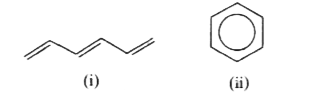 B
B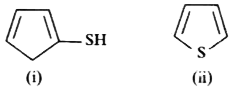 C
C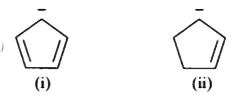 D
D
Which of the following option show more entropy of first compound than second:
A(i) 1-hexene at 273 K and 1 atm (ii) Cyclohexane at 273 K and 1 atmB(i)12CH4 at NTP (ii) 14CH4 at NTPC(1)SO2(g)at300 K and 0.1 atm (ii)SO2(g)at300∘ C and 0.1 atmD(i)C2H5OH(l)at46∘C and 1 atm (ii)CH3OH(l)at46∘ C and 1 atm
In which of the following the first orbital has higher energy than the second in H-aotm?
View SolutionIn which of the following pairs, the first atom is larger than the second ?
View SolutionIn which of the following pairs, the first atom or ion is not large than the second?
View SolutionIn which of the following amines, the first has lower pKb value than the second ?
View SolutionWhich of the following has highest Entropy
View Solution

