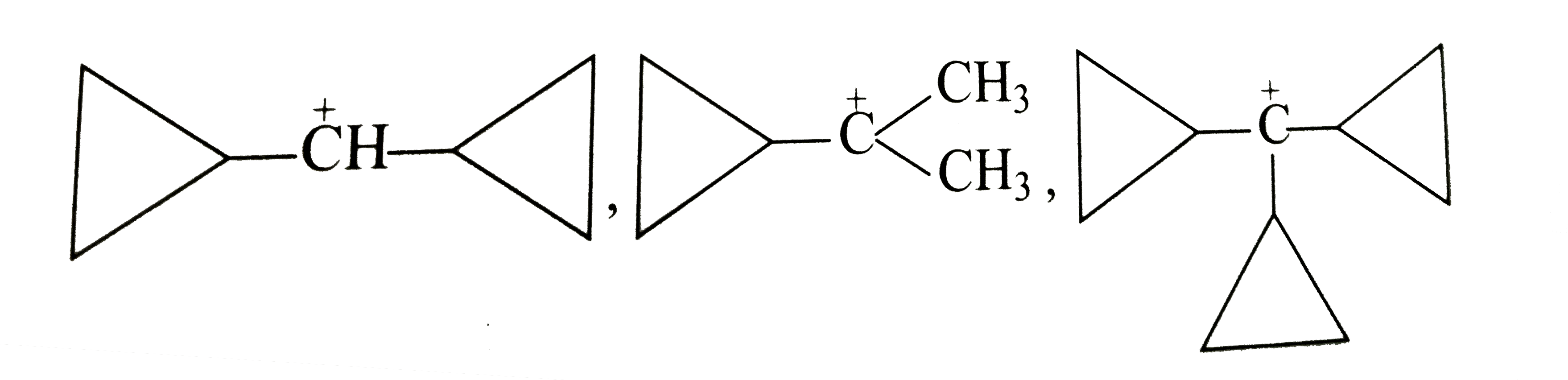
Arrange the following carbocation according to stability
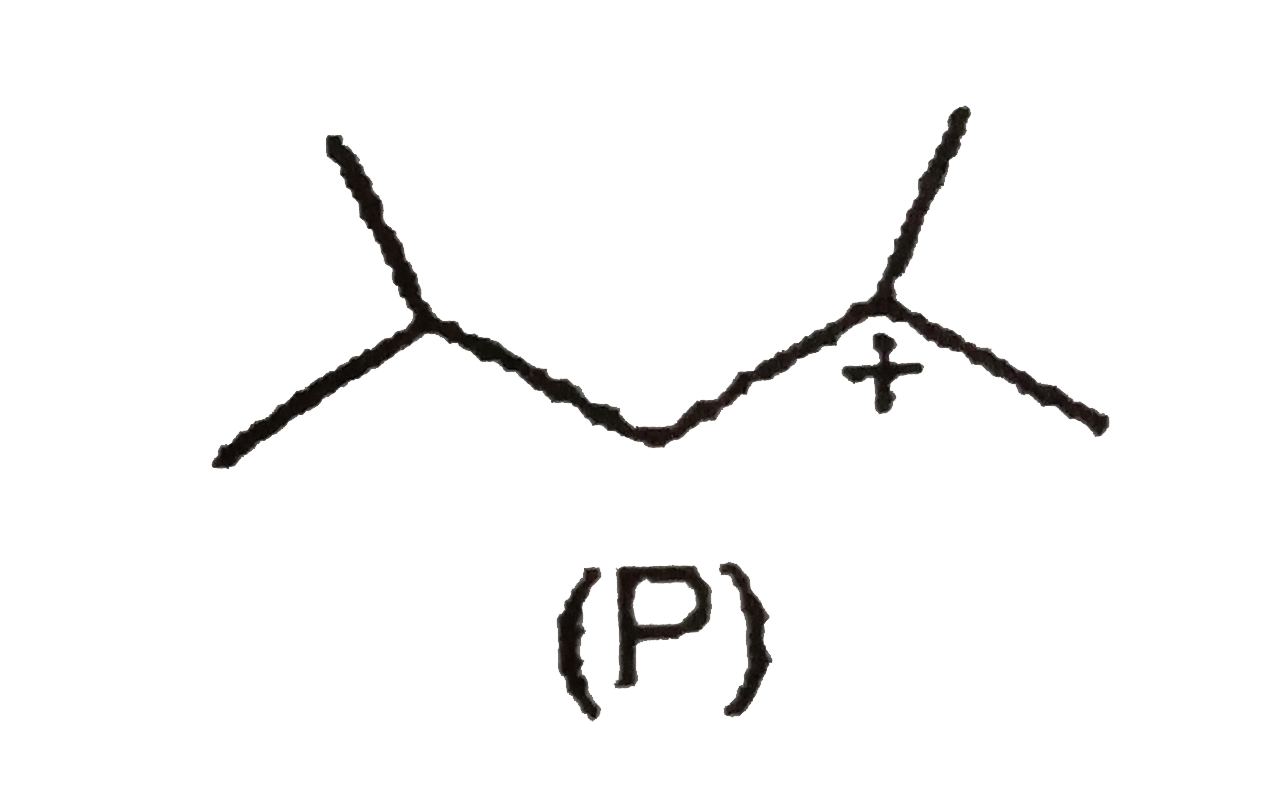
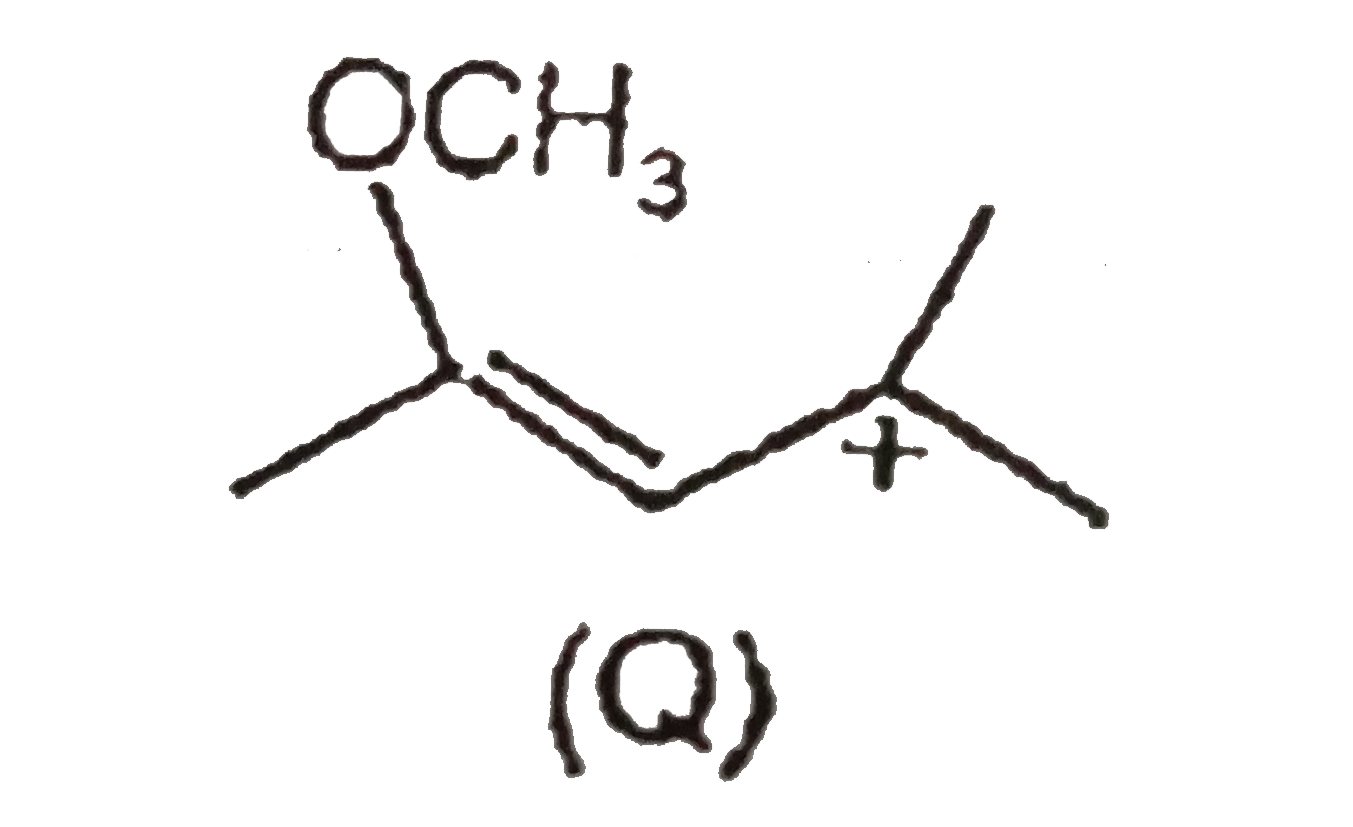
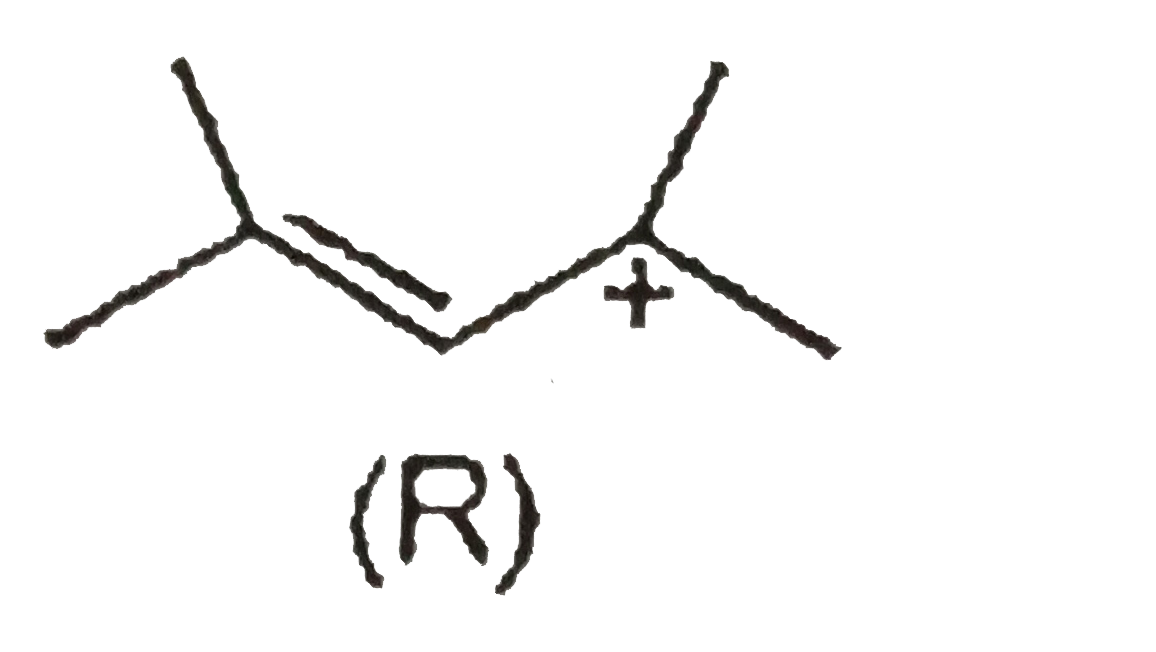
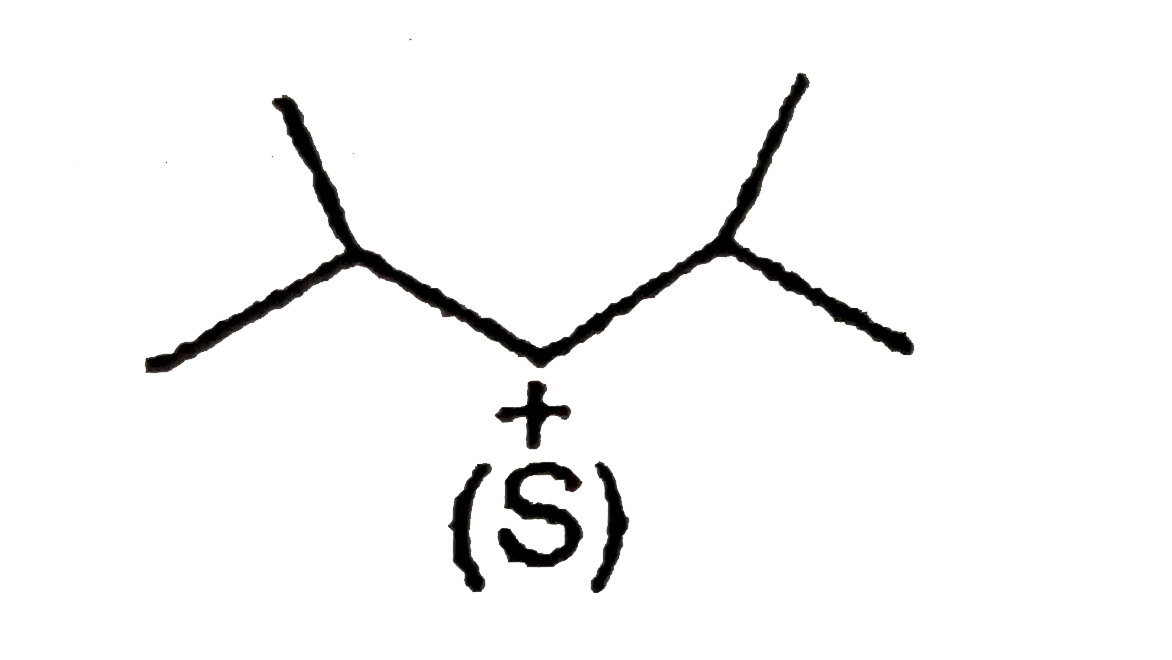
A
R>Q>S>P
B
Q>R>P>S
C
Q>R>S>P
D
R>Q>P>S
Text Solution
The correct Answer is:C
First electron gain enthalpy of all elements is negative except N & Be.
|
Similar Questions
Knowledge Check
Arrange the following carbocation in order of stability :
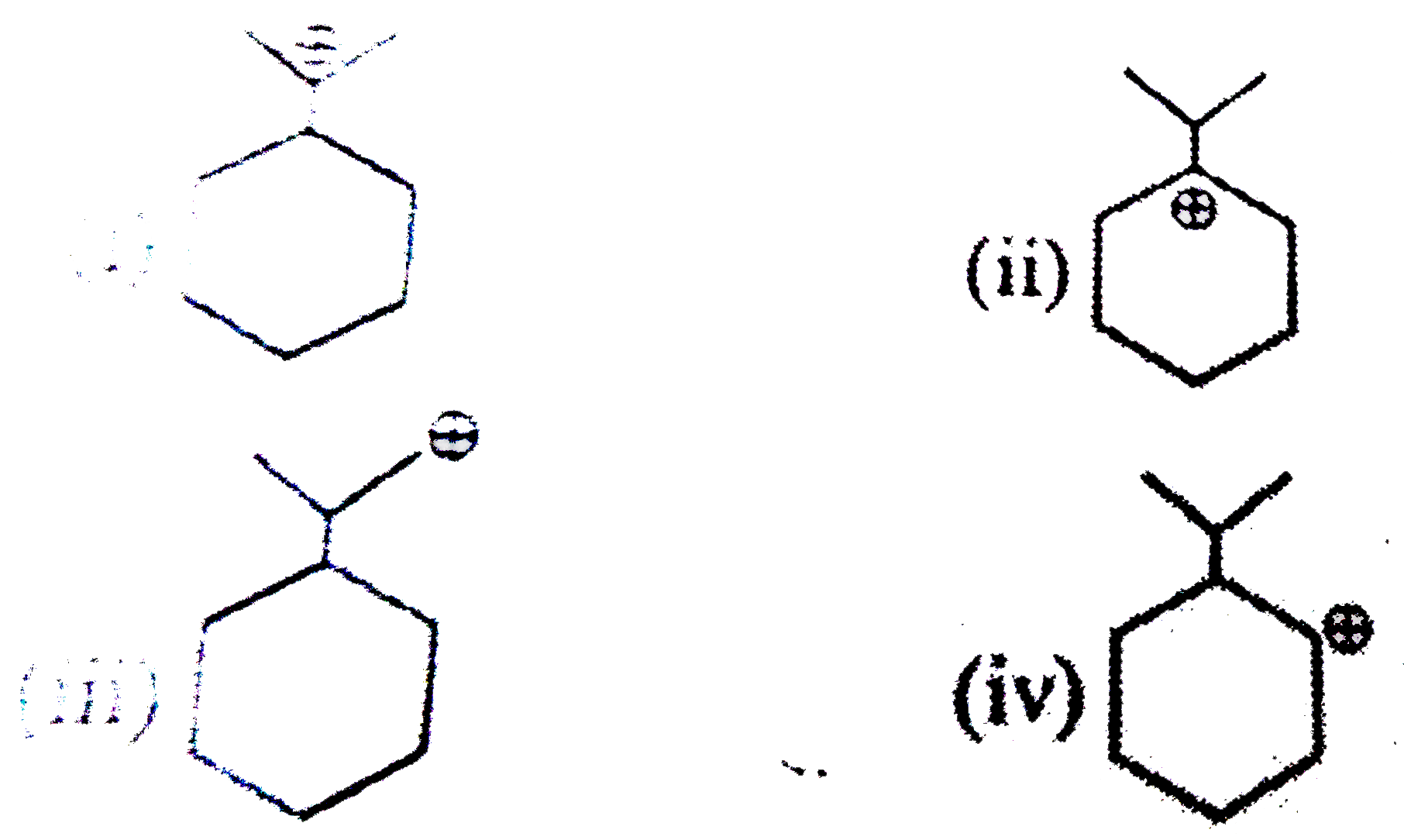 Ai>ii>iii>ivBi>ii>iv>iiiCii>i>iv>iiiDii>i>iii>iv
Ai>ii>iii>ivBi>ii>iv>iiiCii>i>iv>iiiDii>i>iii>ivArrange the following carbocations according to their stability order
(I)C6H5C+H2
(II) C6H5CH2CH2
(III) C6H5C+HCH3
(IV) C6H5+C(CH3)2A(II)<(I)<(III)<(IV)B(I)<(II)<(III)<(IV)C(III)<(II)<(I)<(IV)D(IV)<(I)<(III)<(I)Arrange the following carbocation in decreasing order of stability.
 AIII>I>IV>IBIII>II>I>IVCI>III>II>IVDIII>I>II>IV
AIII>I>IV>IBIII>II>I>IVCI>III>II>IVDIII>I>II>IV
Arrange the followinng carbocations in decreasing order of stability
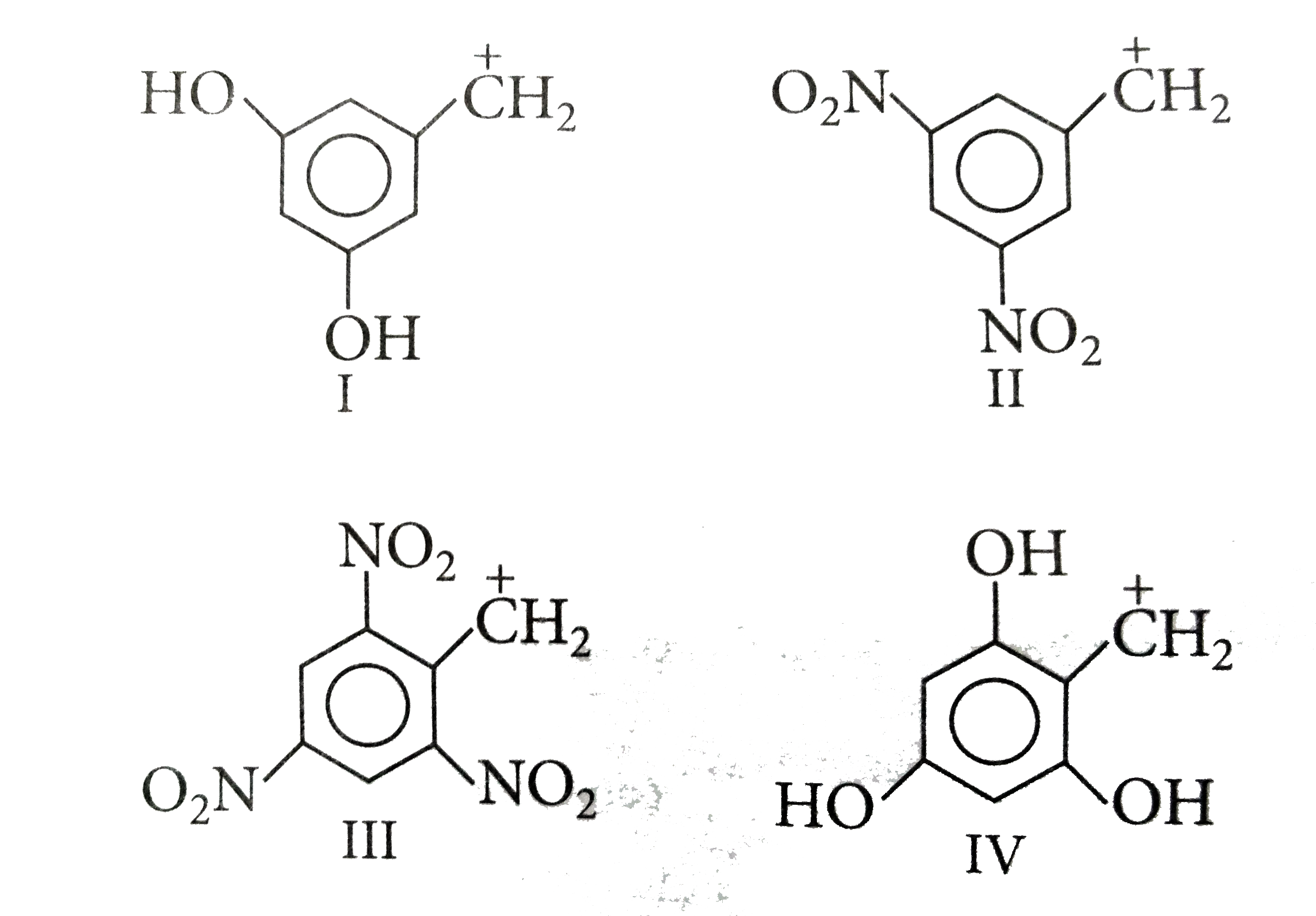
View SolutionArrange the given carbocation in decreasing order of stability :
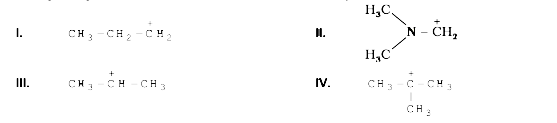
The correct choice is :
View SolutionArrange the following carbocation in decreasing order of stability.

View SolutionArrange the following carbocations in order of stability
benzyl allyl methyl vinyl I II III IV
View SolutionIn the following carbocations, the stability order is:

View Solution