Explain the mechanism of esterification carboxylic acids.
Video Solution
Text Solution
Mechanism of estrification of carboxylic acids : It is a kind of nucleophilhc acyl substitution. The mechanism of esterification involves the following step:
Step I. Protonation of the carbonyl group.
In presence of mineral acids (conc. H2SO4orHCl) the carbonyl oxygen of carboxylic acid accepts a proton to,form protonated carboxylic acid (I).
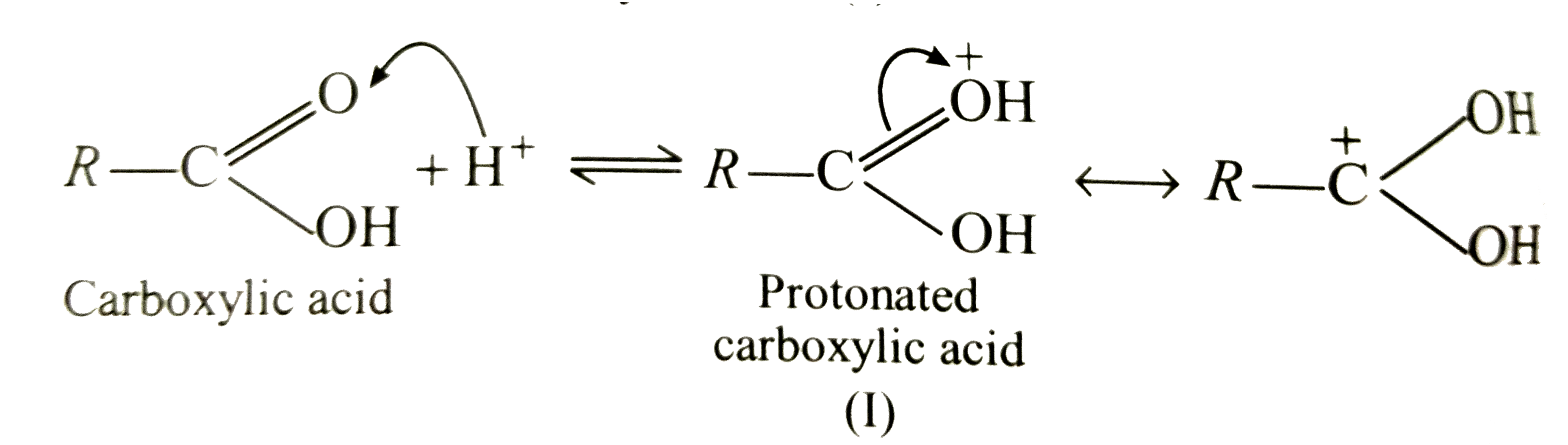
Step II. Nucleophilic attack by the alcohol molecule
The electron rich oxygen atom of alcohol molecule attaches itself at positively charged carbon atom to form tetrahedral intermediate (II).
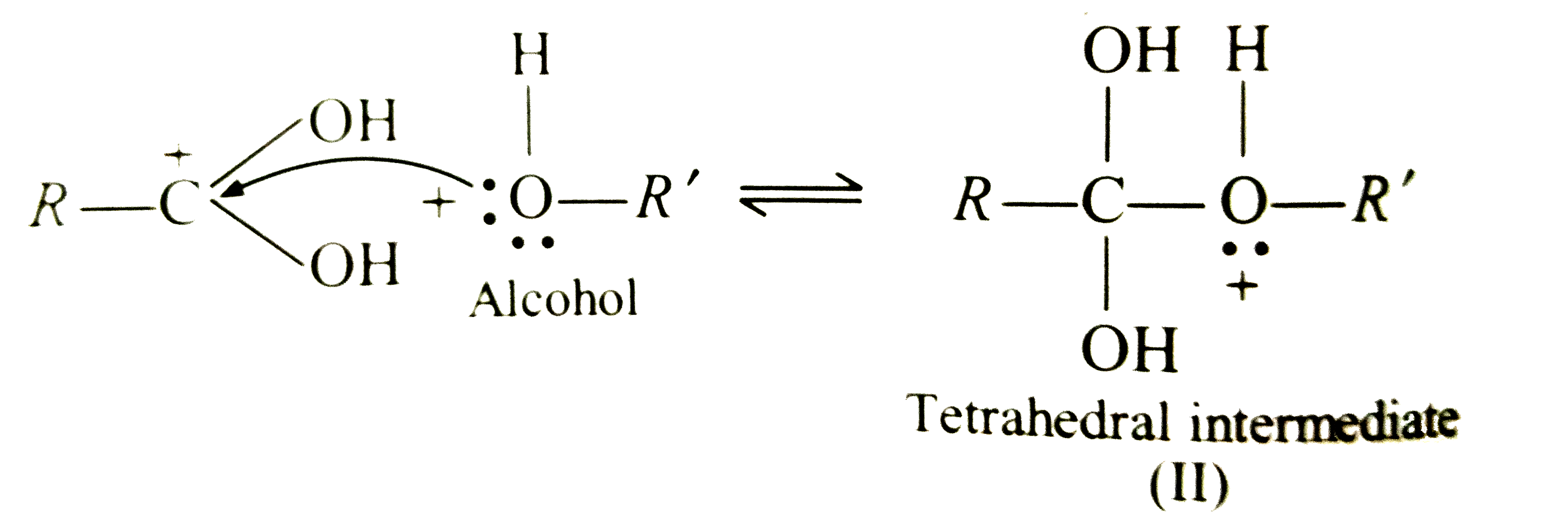
Step Ill. Transfer of proton.
From the resulting intermediate, a proton shifts to -OH and forms another tetrahedral intermediate (Ill).
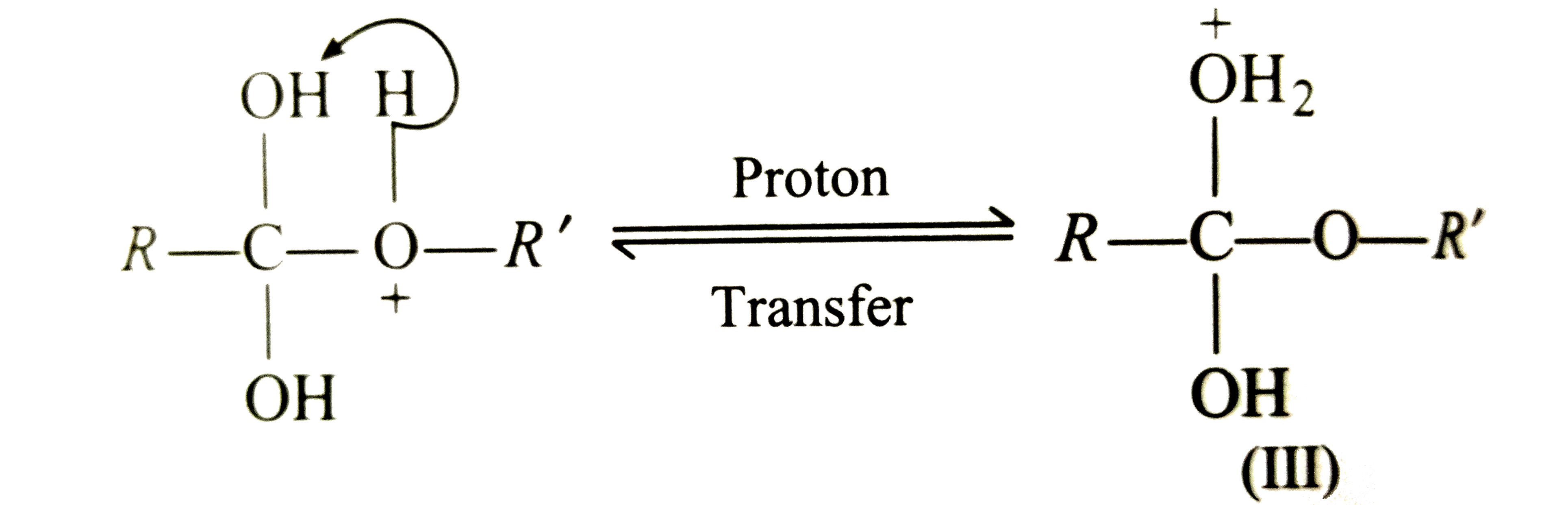
step IV. Loss of water molecule
The intermediate (III) loses a molecule of water to afford protonated ester (IV).
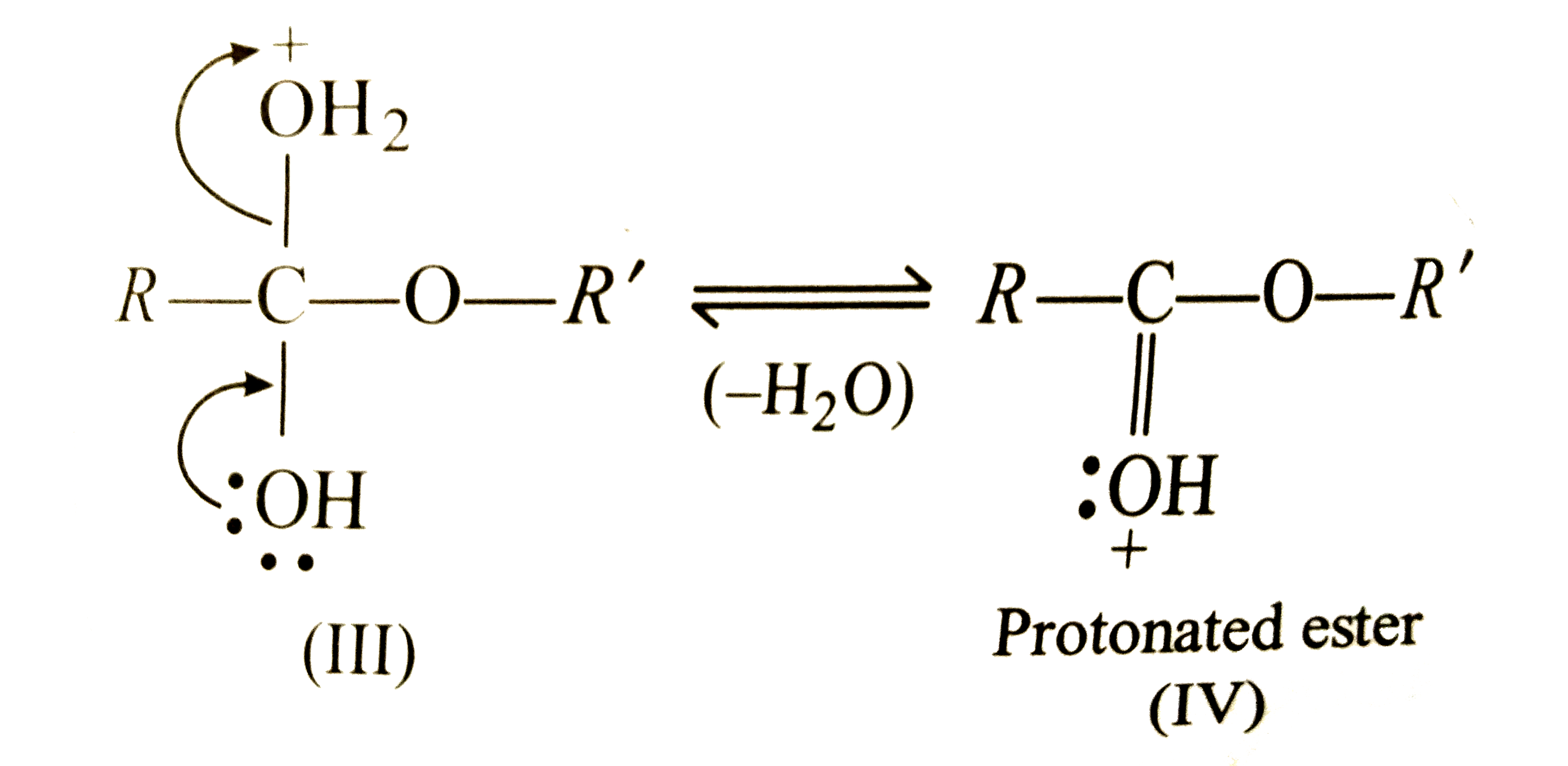
Step v. Loss of proton.
The protonated ester finally loses a proton to form an ester
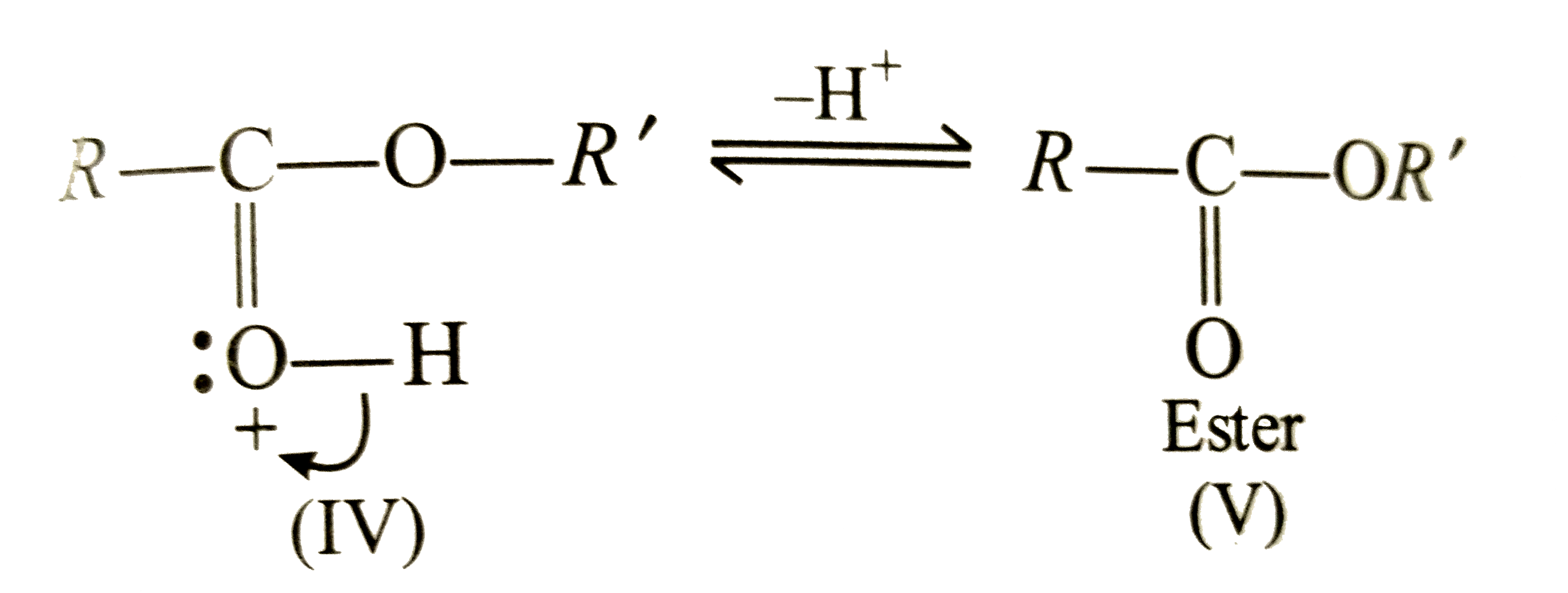
The mechanism is supported by the isotropic tracer studies that esterification involves the cleavage of H of O- H bond of alcohols and -OH of - CO- OH bond of carrboxylic acids as suggested by the above mechanism. For example, when acetic acid is esterified with isotopically labelled methanol (CH318OH), the ester (i.e., methyl acetate) thus formed was found to contain 18O while water formed did not have any isotopic oxygen.
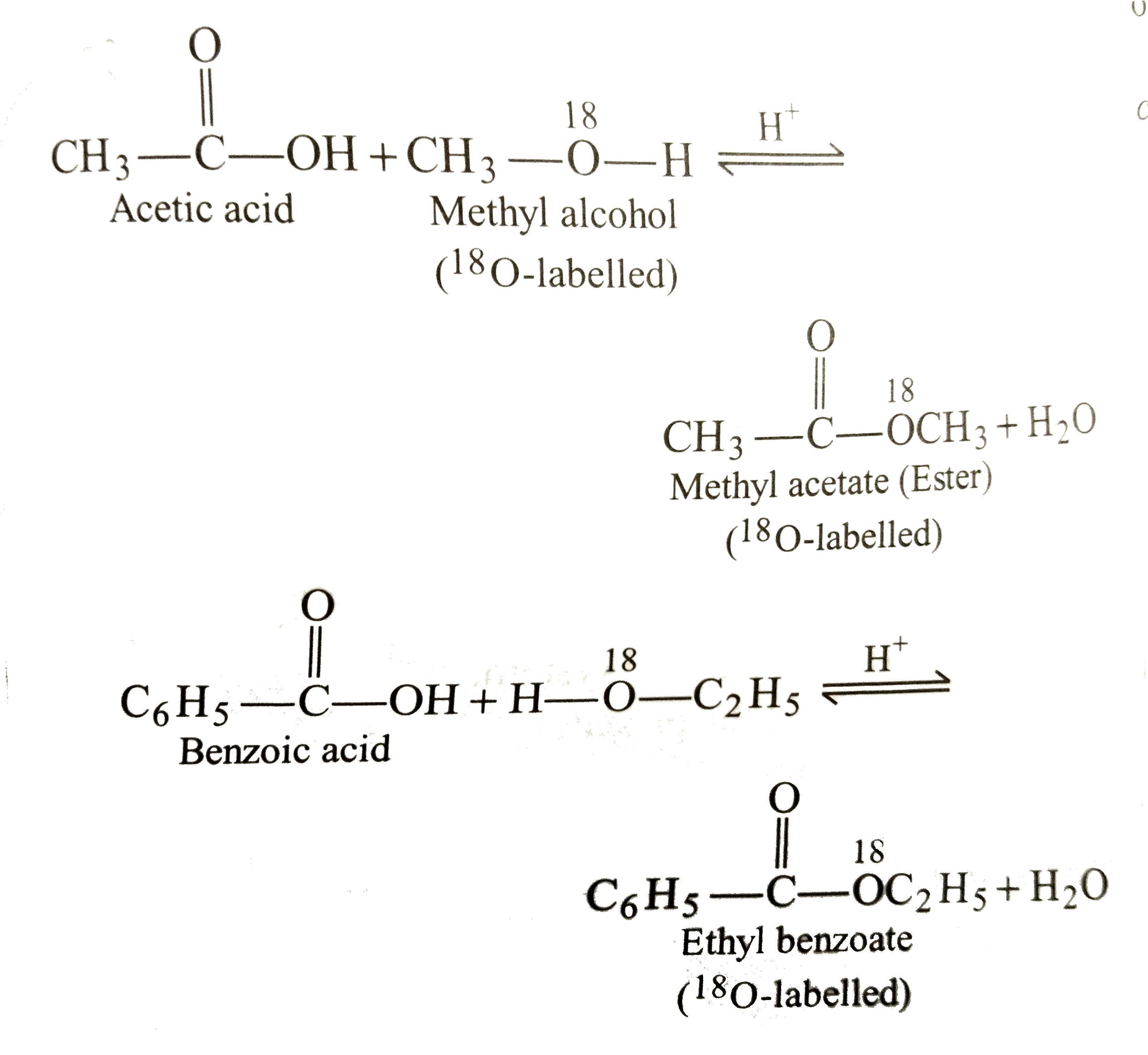
|
Similar Questions
Knowledge Check
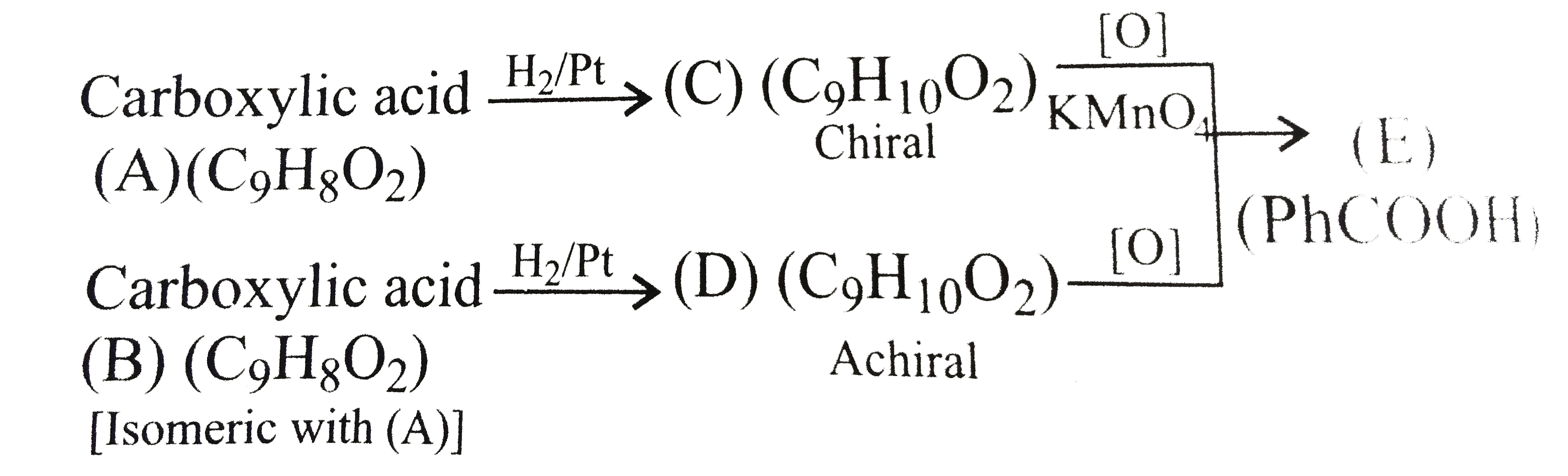
Carboxylic acid (A) is:A B
B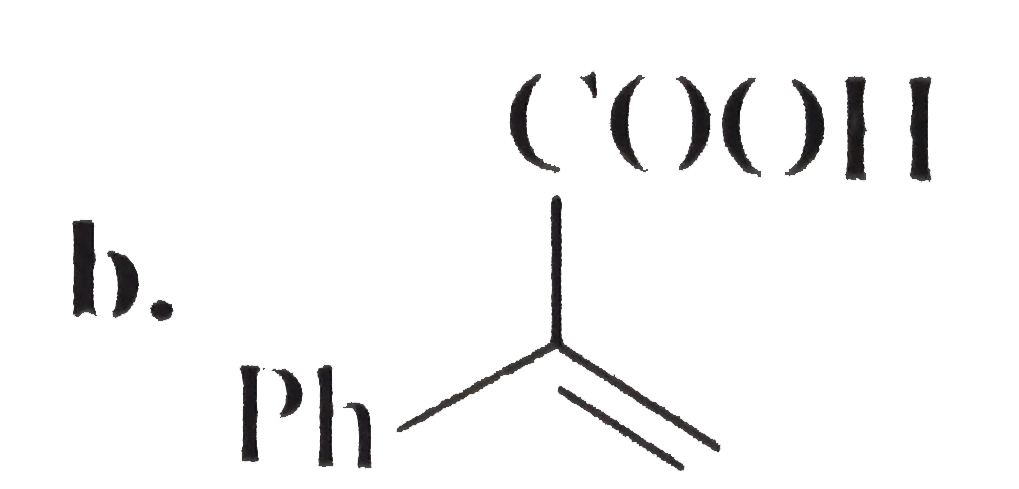 C
C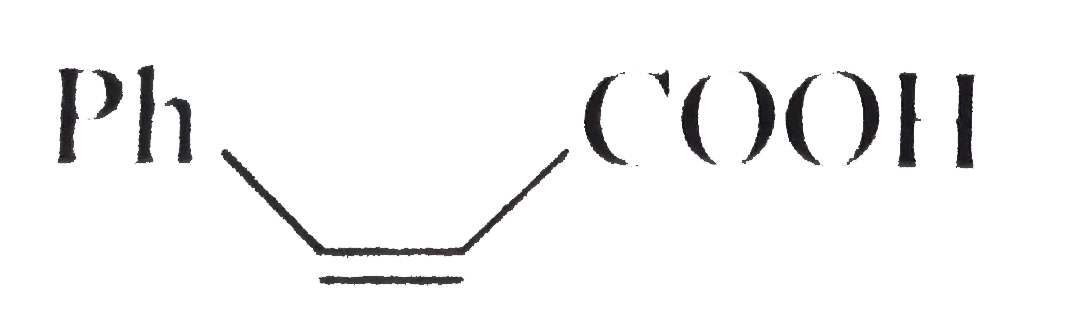 DBoth (a) and (c )
DBoth (a) and (c )Which of the following intermediates are involved in the acid catalyzed esterification of carboxylic acid?
A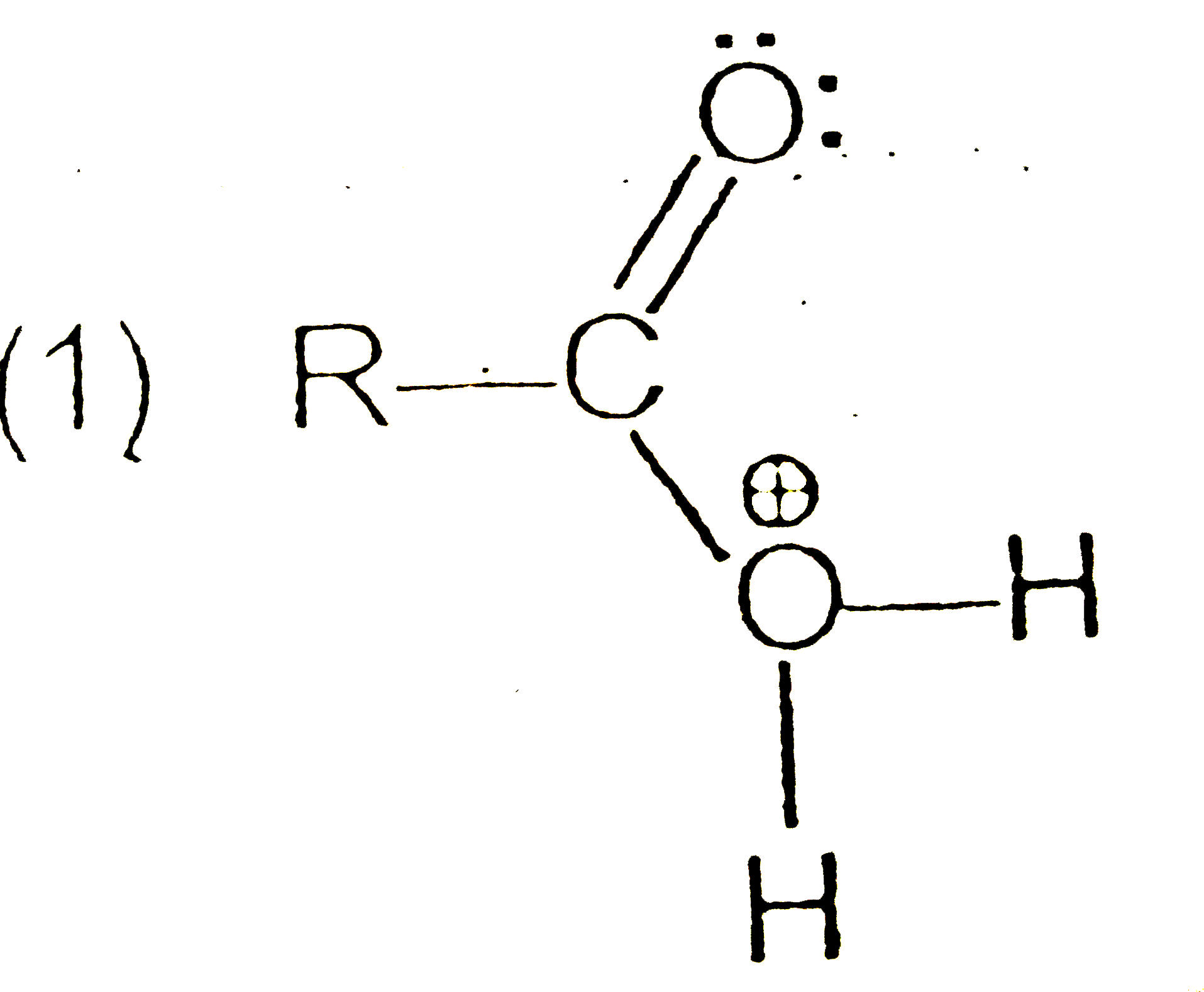 B
B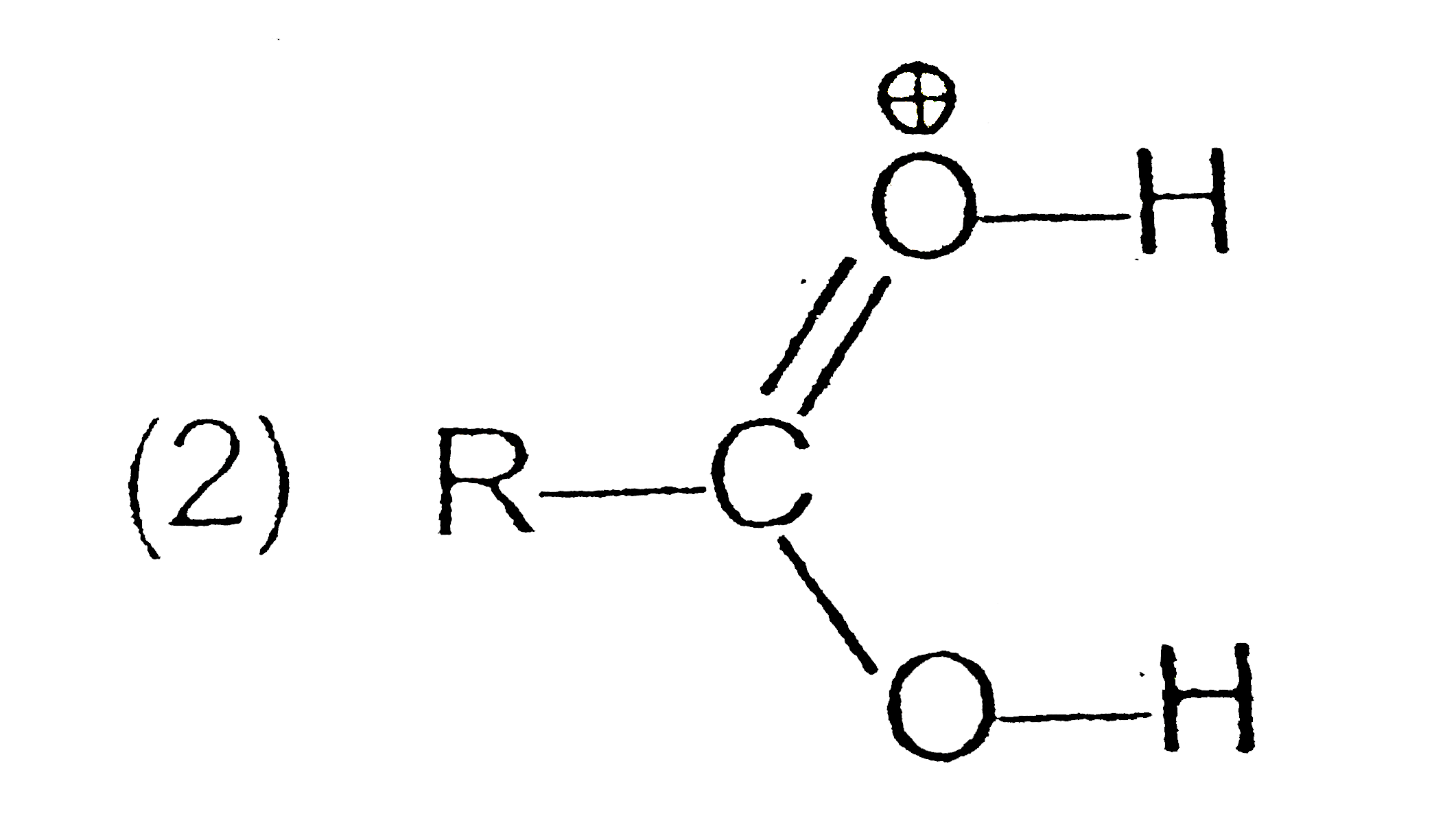 C
C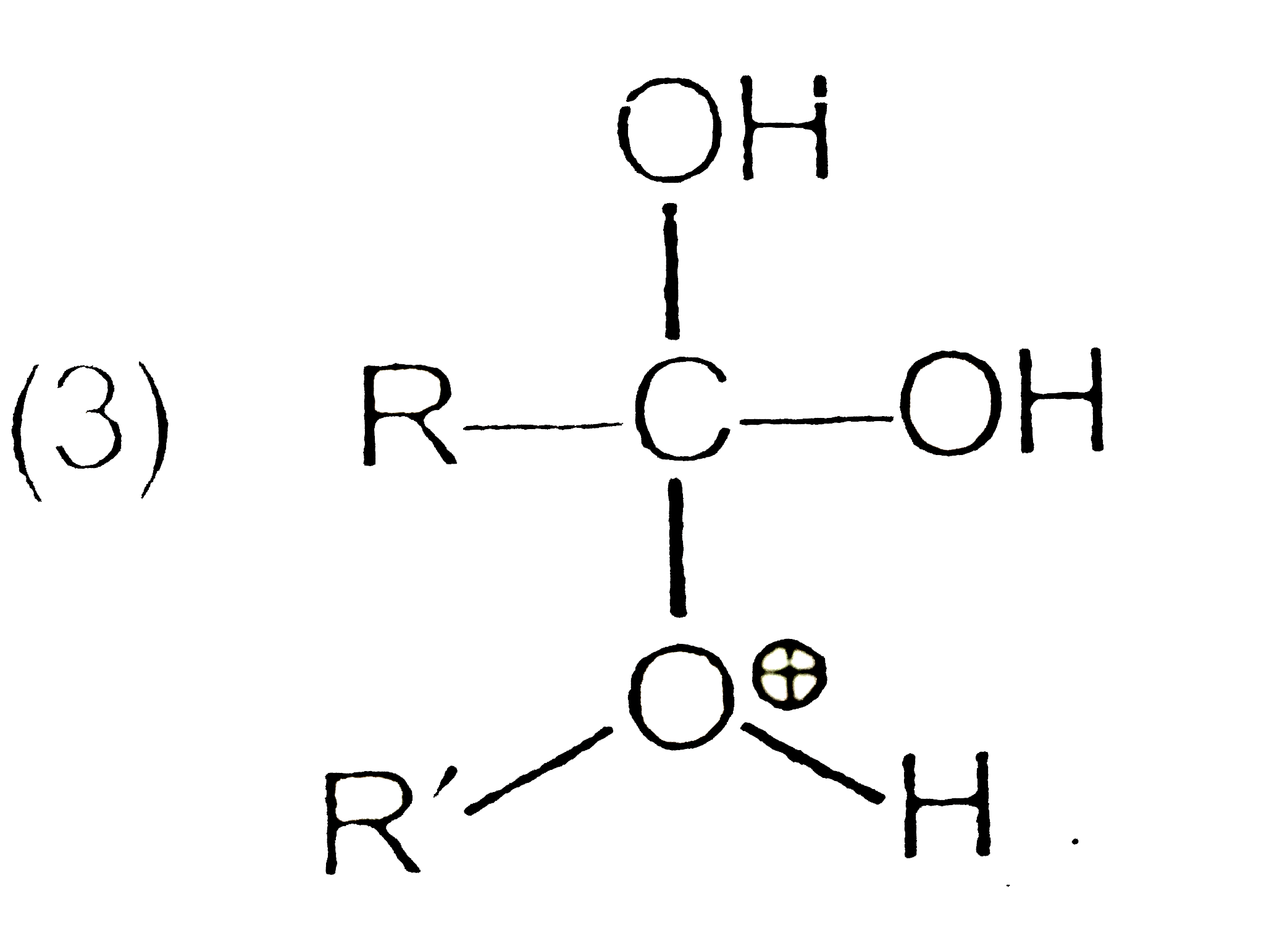 D
D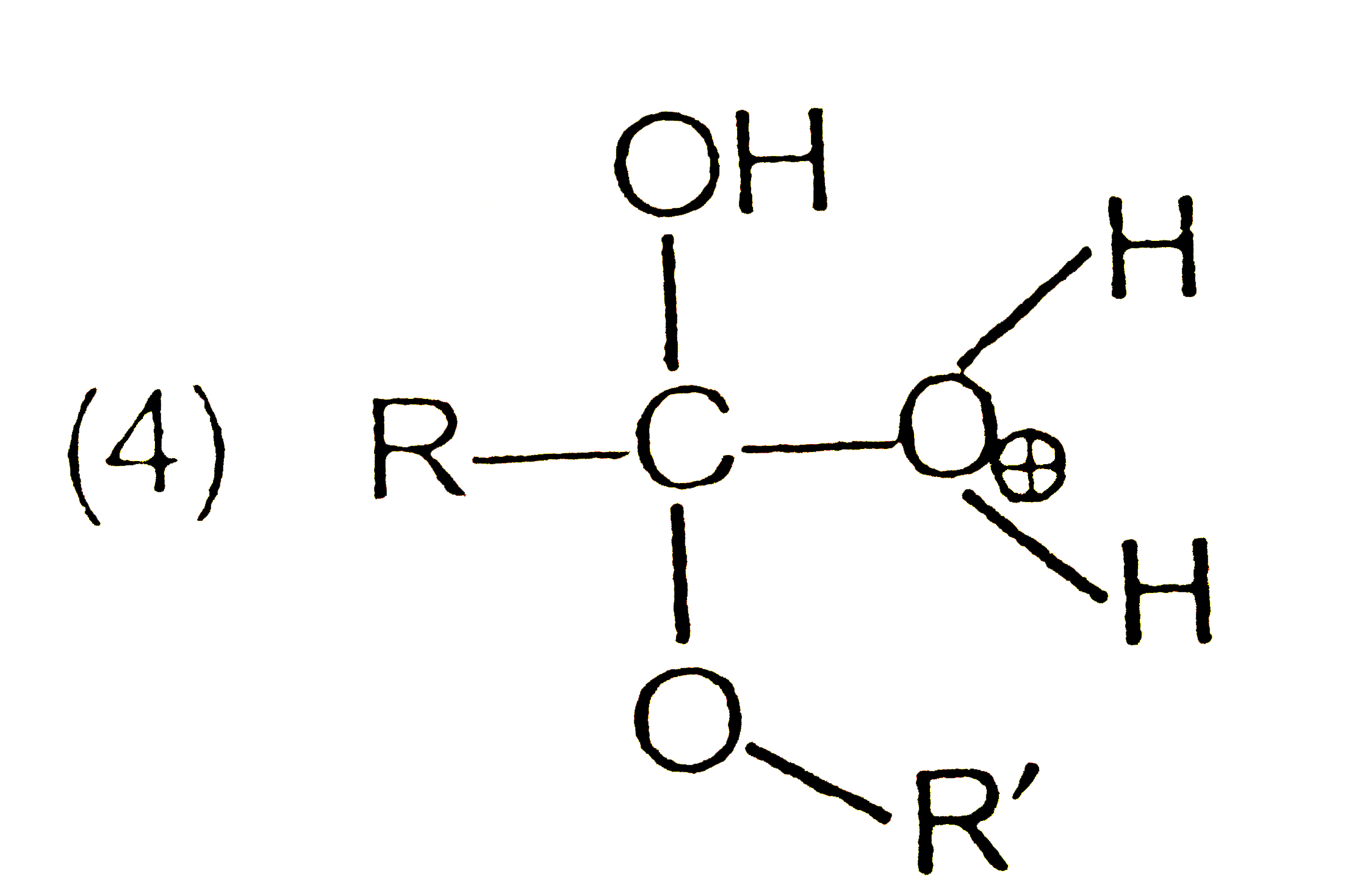
The isomers of carboxylic acids are
AalcoholsBacid aminesCaldehydesDesters
Explain the mechanism of esterification. Write the reactions involved in dehydration of 1∘,2∘ and 3∘ alcohols
View SolutionPreparation of Carboxylic acids
View SolutionDraw structure of salicylaldehyde.
Write reaction for preparation of acetophenone from benzoyl chloride.
Explain the acidic nature of carboxylic acids.
View SolutionThe mechanism of esterification in presence of acid catalyst (H2SO4) is proposed as follows:
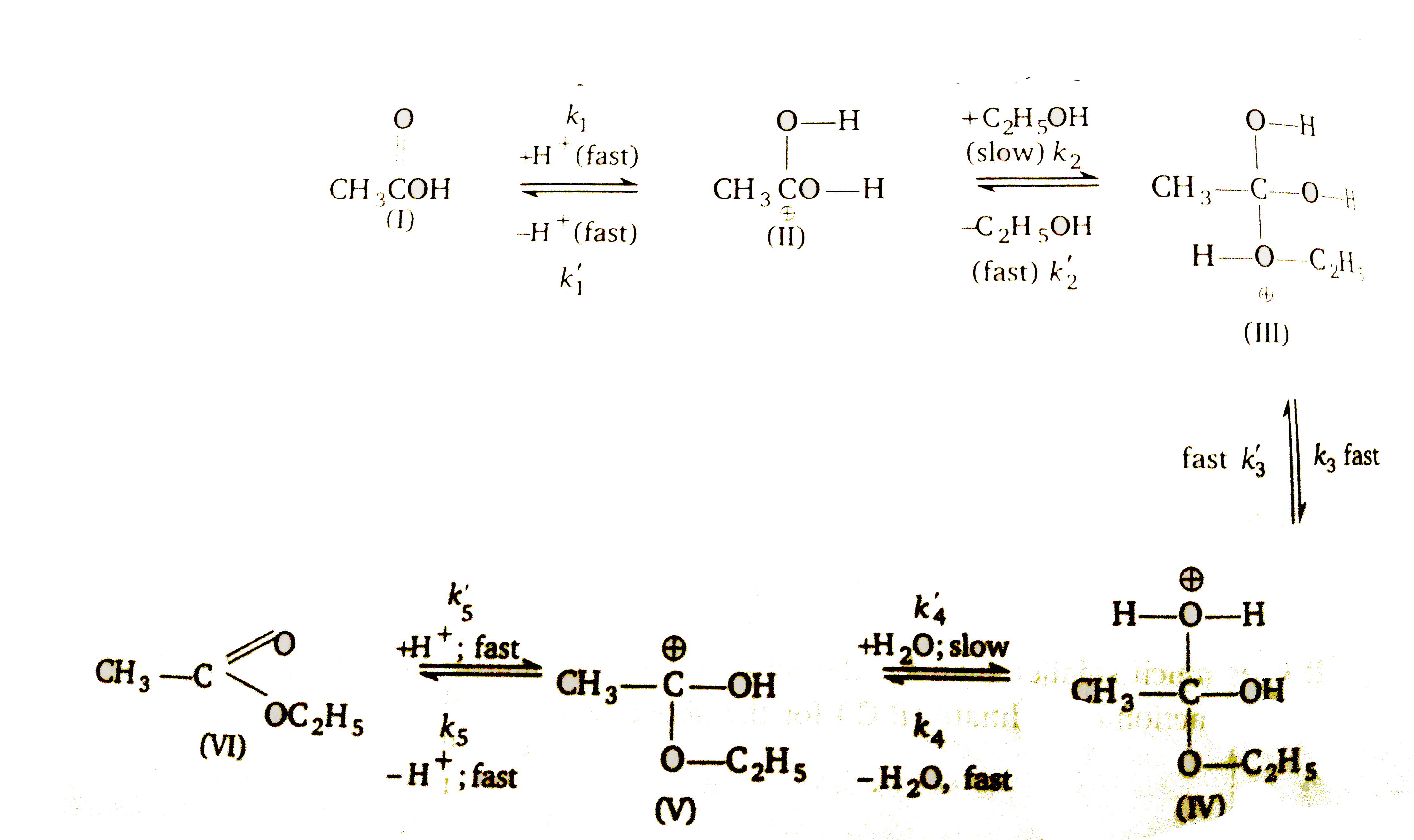
Which of the following potential energy Vs reaction co-ordinate diagram is consitent with given mechanism ?
View SolutionIn the esterification of alcohol by carboxylic acid, proton is given by-
View Solution
