Explain the mechanism of esterification of carboxylic acid. (Feb. ' 16)
Text Solution
The esterification of carboxylic acids with alcohols is a nucleophilic acylic substitution . The carboxylic acid is protonated on its carboxyl oxygen atom . Alcohol acts as a nucleophilic and attacks carboxyl carbon . Loss of proton gives ester hydrate.
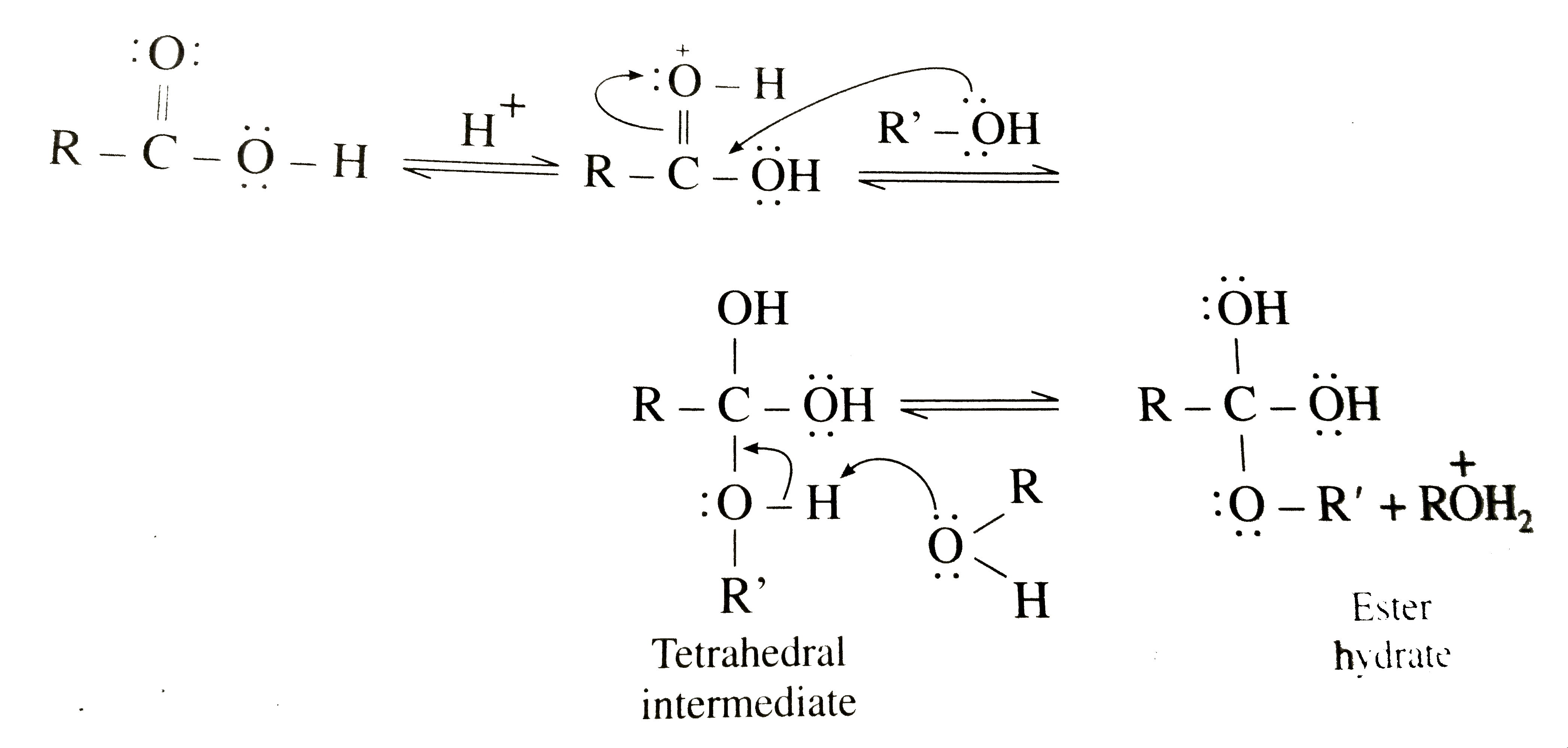
Further , one of the -OH group of ester hydrate gets protnated and water molecule departs.
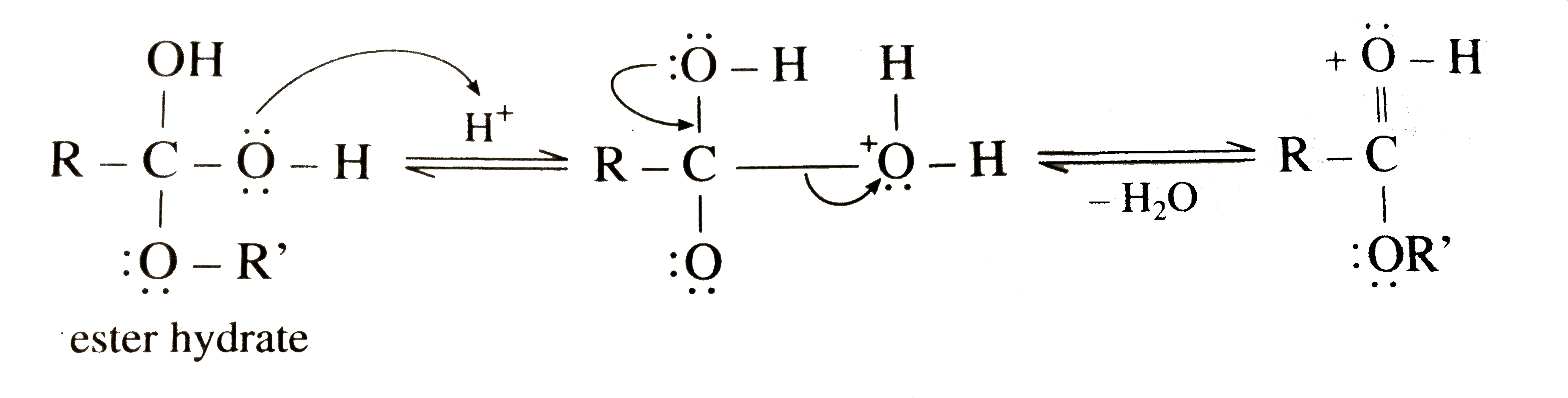
The loss of proton from second hydroxyl group gives ester.
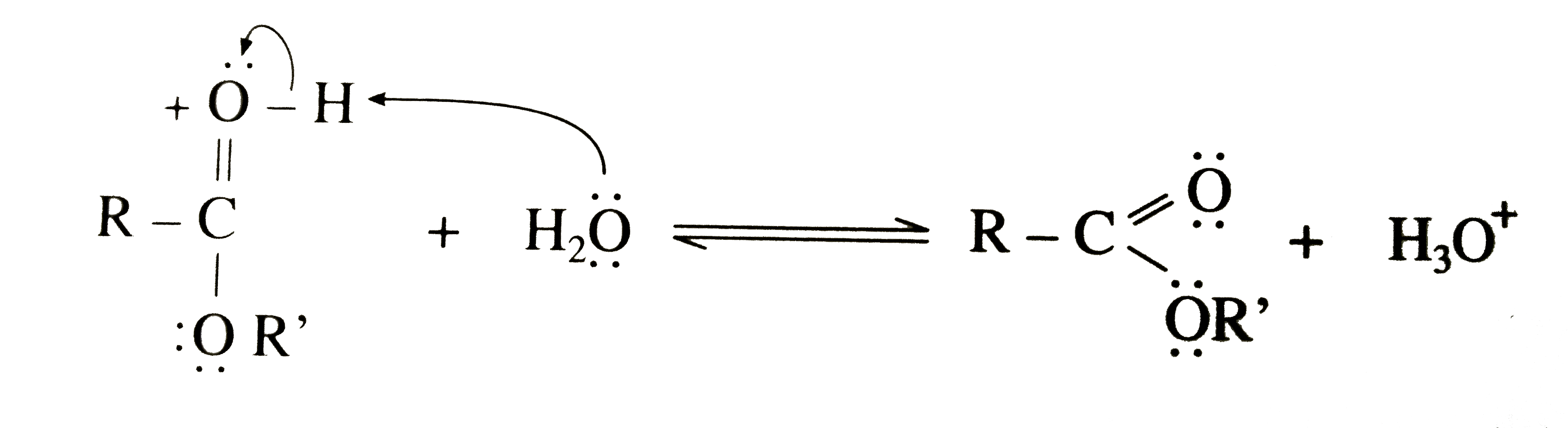
In esterification , water molecule is formed by the combination of H - atom of the alcohol and - OH group of the carboxylic acid.
|
Similar Questions
Knowledge Check
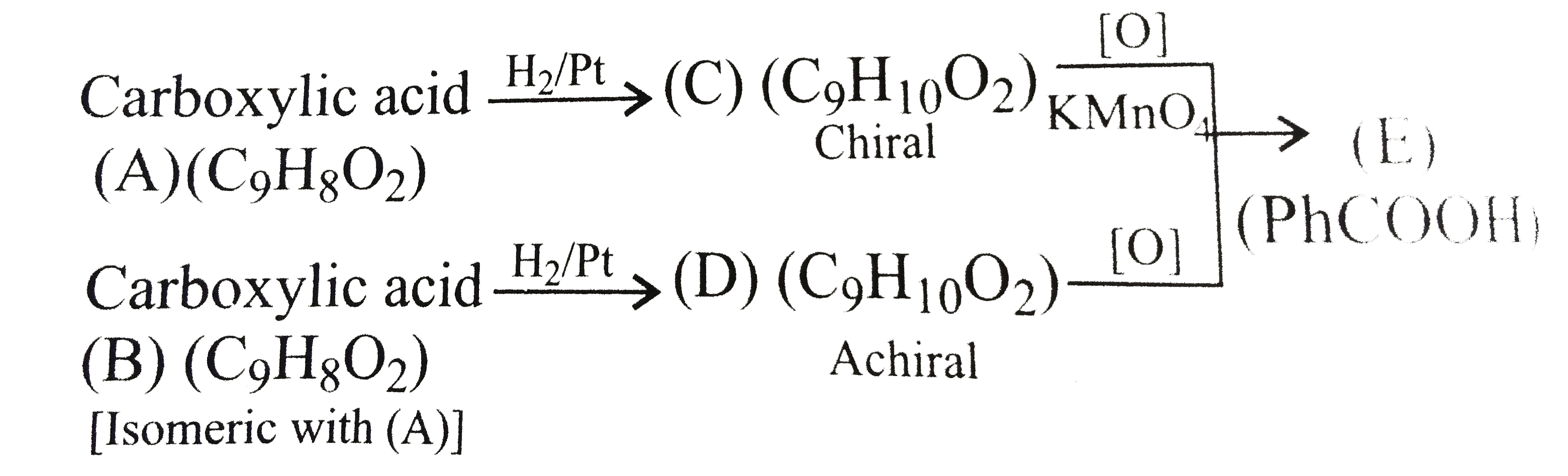
Carboxylic acid (A) is:A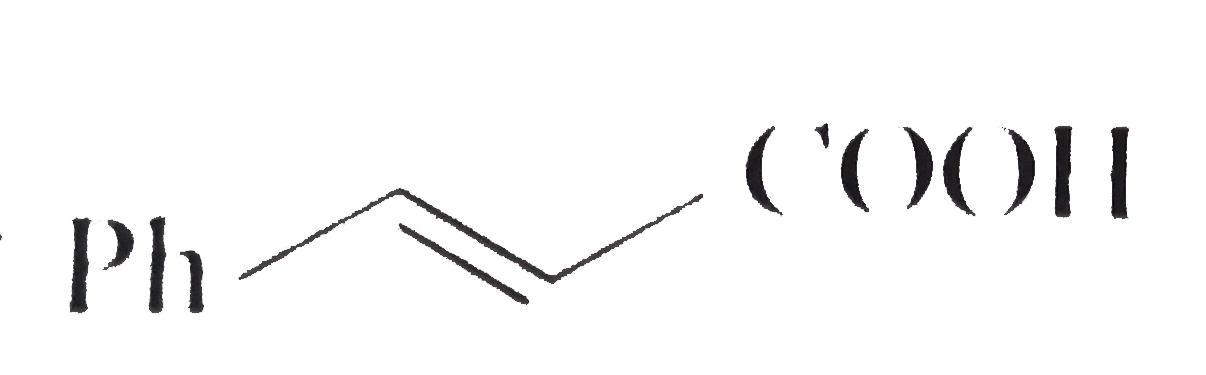 B
B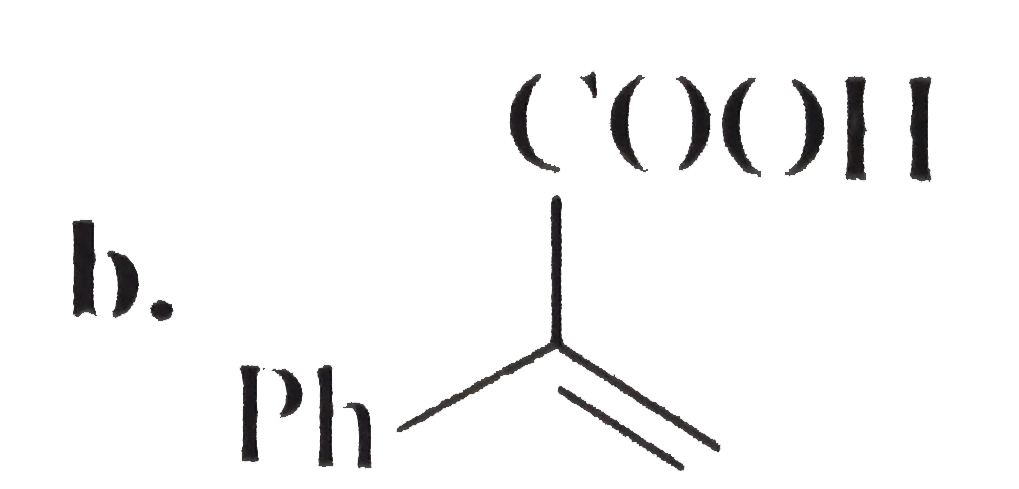 C
C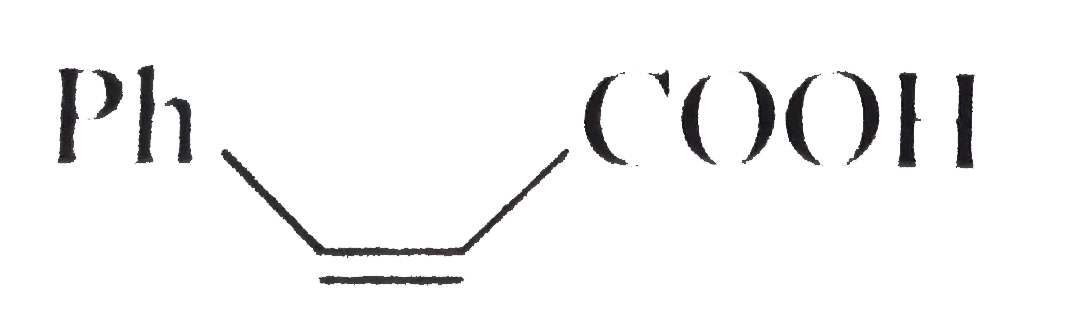 DBoth (a) and (c )
DBoth (a) and (c )The isomers of carboxylic acids are
AalcoholsBacid aminesCaldehydesDestersWhich of the following intermediates are involved in the acid catalyzed esterification of carboxylic acid?
A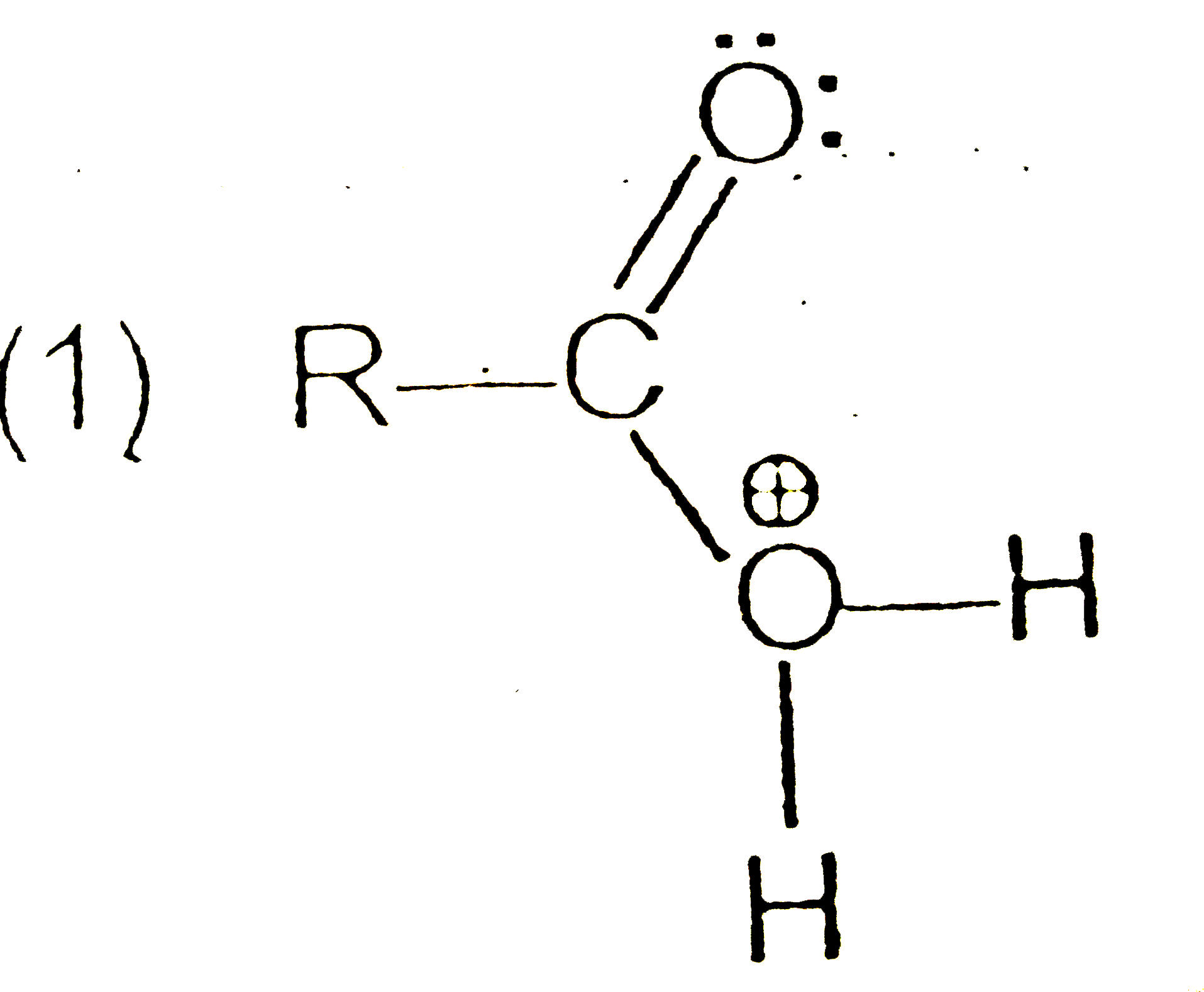 B
B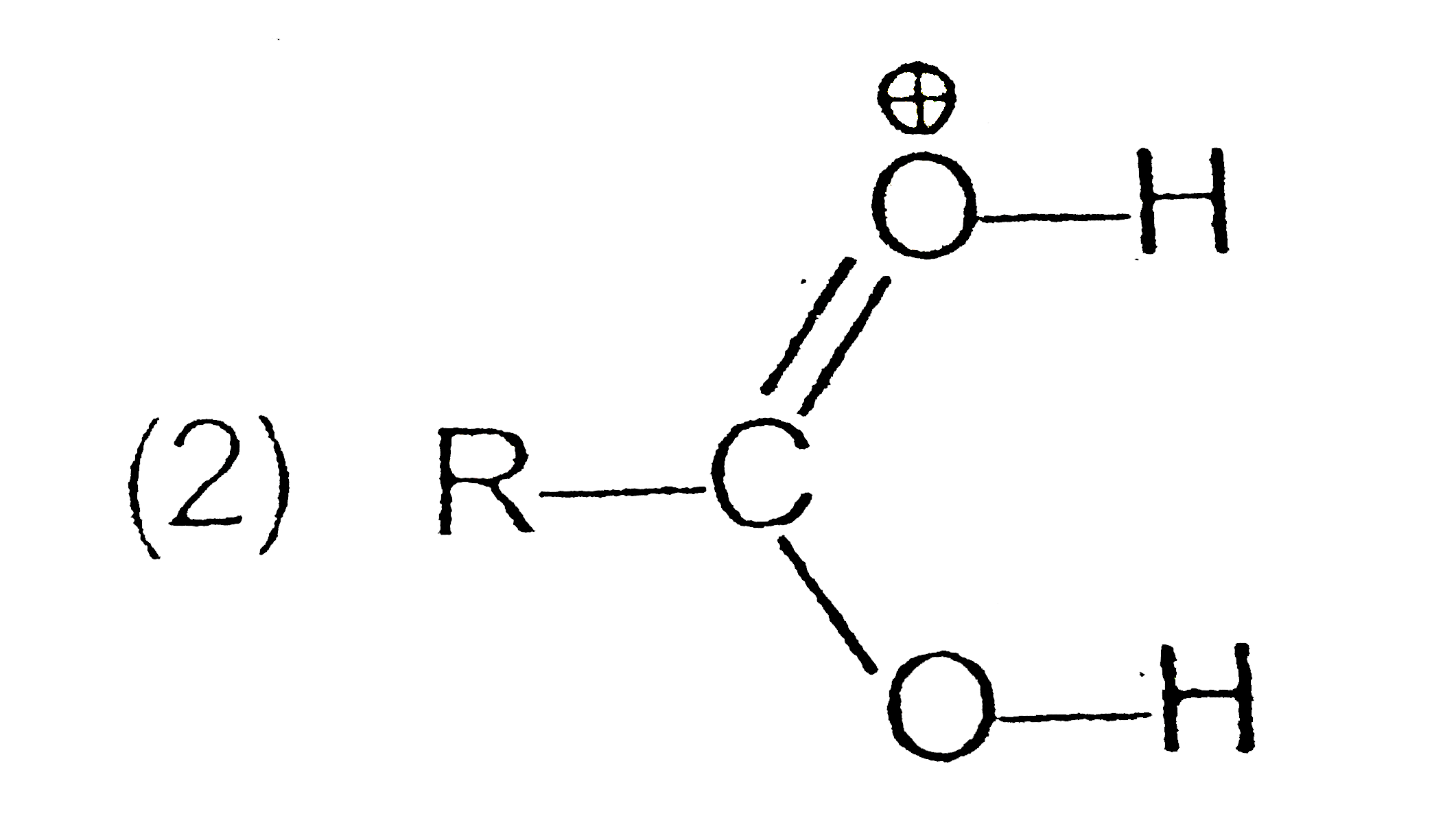 C
C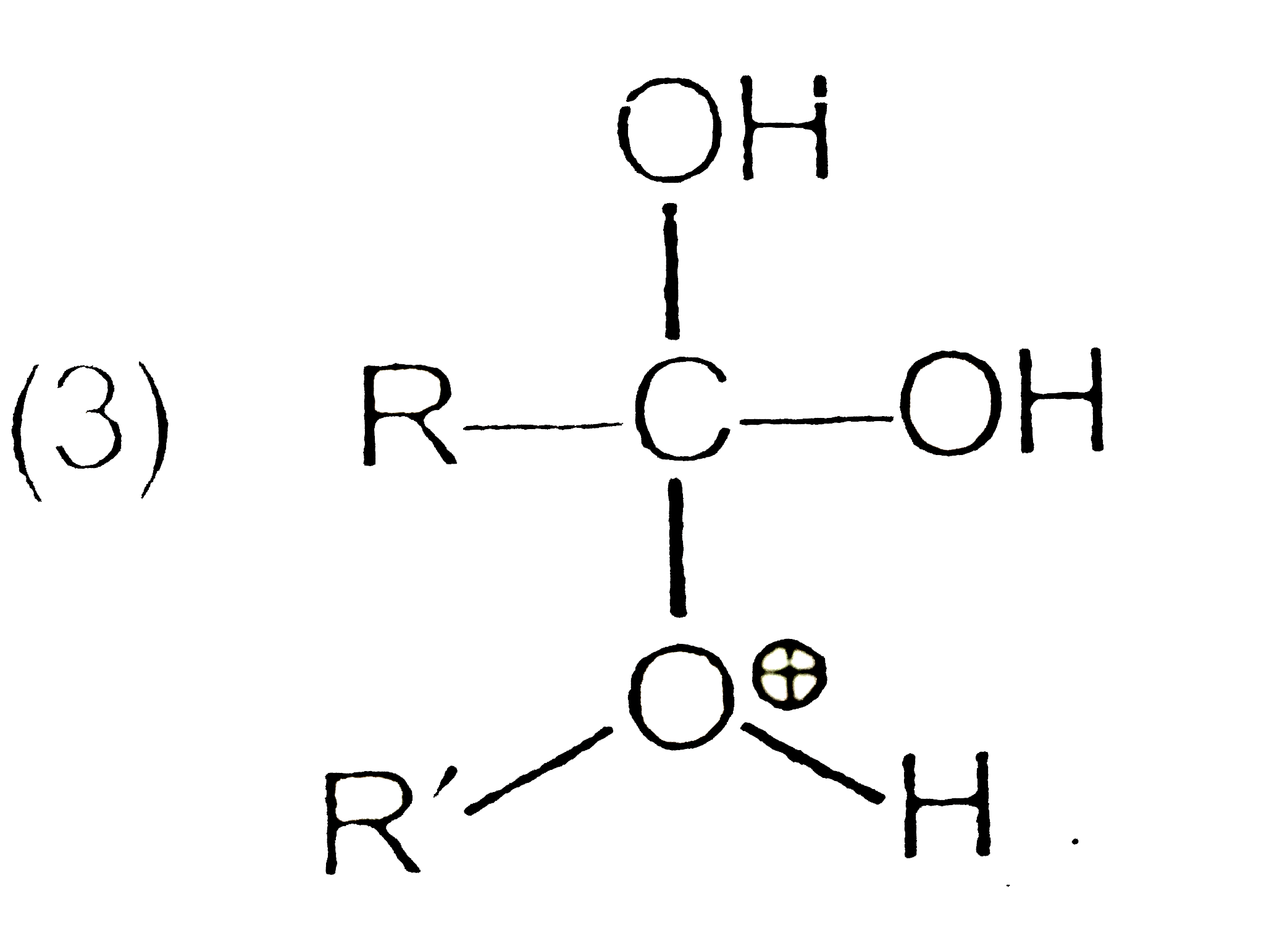 D
D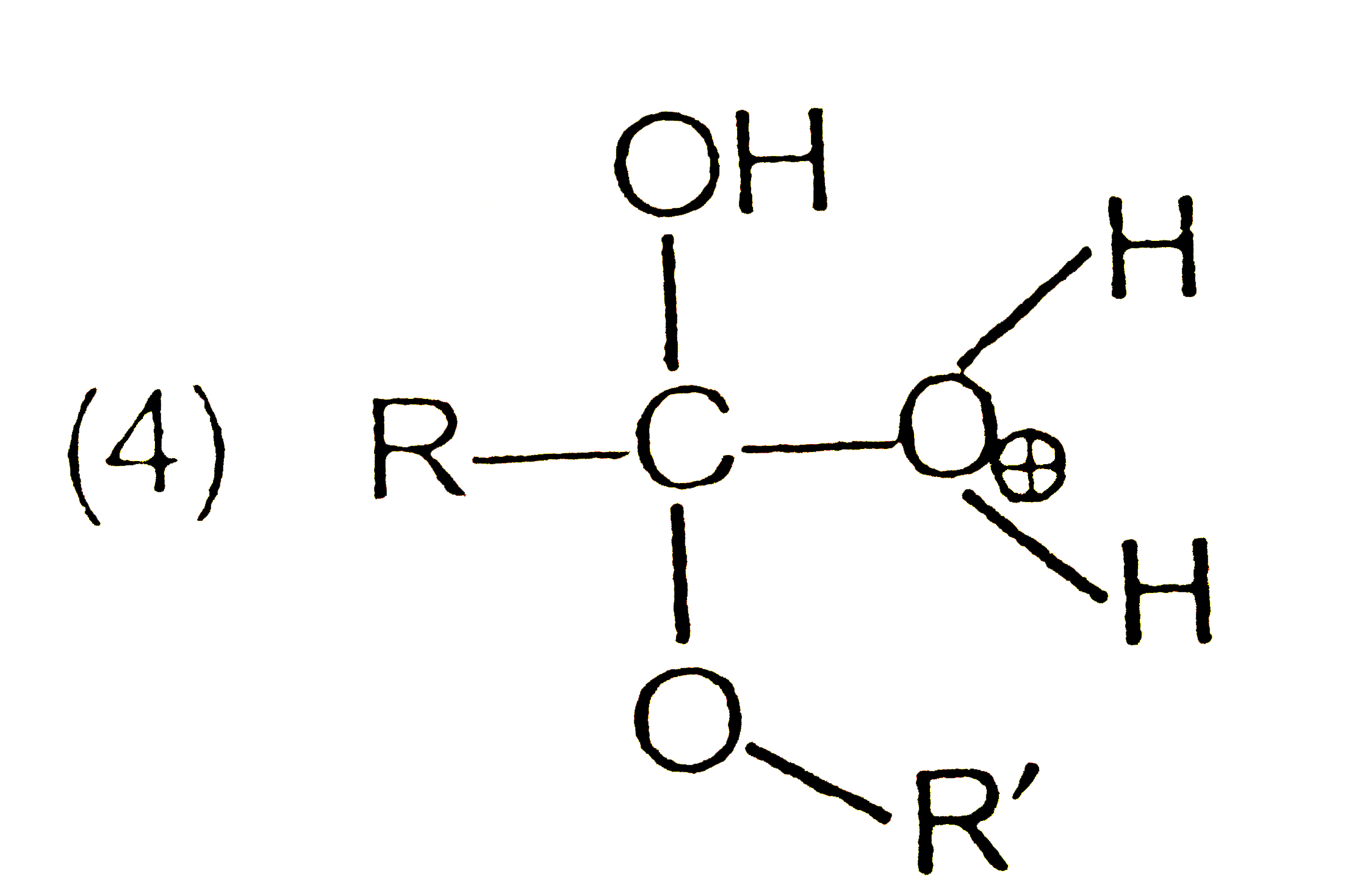
Carboxyllic Acid
View SolutionMethods of preparation of Carboxylic acids
View SolutionExplain the mechanism of esterification. Write the reactions involved in dehydration of 1∘,2∘ and 3∘ alcohols
View SolutionThe esterification of carboxylic acids with alcohols is a kind of
View SolutionIUPAC name of carboxylic acid is
View Solution

